Phosphorelay as the sole physiological route of signal transmission by the arc two-component system of Escherichia coli
- PMID: 10851007
- PMCID: PMC94563
- DOI: 10.1128/JB.182.13.3858-3862.2000
Phosphorelay as the sole physiological route of signal transmission by the arc two-component system of Escherichia coli
Abstract
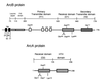
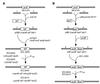
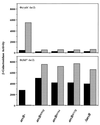
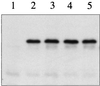
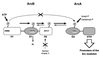
The Arc two-component system, comprising a tripartite sensor kinase (ArcB) and a response regulator (ArcA), modulates the expression of numerous genes involved in respiratory functions. In this study, the steps of phosphoryl group transfer from phosphorylated ArcB to ArcA were examined in vivo by using single copies of wild-type and mutant arcB alleles. The results indicate that the signal transmission occurs solely by His-Asp-His-Asp phosphorelay.
Figures
Similar articles
-
Routes of phosphoryl group transfer during signal transmission and signal decay in the dimeric sensor histidine kinase ArcB.J Biol Chem. 2018 Aug 24;293(34):13214-13223. doi: 10.1074/jbc.RA118.003910. Epub 2018 Jun 26. J Biol Chem. 2018. PMID: 29945971 Free PMC article.
-
The SixA phospho-histidine phosphatase modulates the ArcB phosphorelay signal transduction in Escherichia coli.FEBS Lett. 2000 Mar 24;470(2):118-24. doi: 10.1016/s0014-5793(00)01303-x. FEBS Lett. 2000. PMID: 10734219
-
Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo.J Bacteriol. 2005 May;187(9):3267-72. doi: 10.1128/JB.187.9.3267-3272.2005. J Bacteriol. 2005. PMID: 15838055 Free PMC article.
-
Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression.Antioxid Redox Signal. 2006 May-Jun;8(5-6):781-95. doi: 10.1089/ars.2006.8.781. Antioxid Redox Signal. 2006. PMID: 16771670 Review.
-
Signal transduction via the histidyl-aspartyl phosphorelay.Genes Cells. 1997 Mar;2(3):167-84. doi: 10.1046/j.1365-2443.1997.d01-311.x. Genes Cells. 1997. PMID: 9189755 Review.
Cited by
-
Cross Talk Inhibition Nullified by a Receiver Domain Missense Substitution.J Bacteriol. 2015 Oct;197(20):3294-306. doi: 10.1128/JB.00436-15. Epub 2015 Aug 10. J Bacteriol. 2015. PMID: 26260457 Free PMC article.
-
Evolution of the metabolic and regulatory networks associated with oxygen availability in two phytopathogenic enterobacteria.BMC Genomics. 2012 Mar 22;13:110. doi: 10.1186/1471-2164-13-110. BMC Genomics. 2012. PMID: 22439737 Free PMC article.
-
Effect of D-lactate on the physiological activity of the ArcB sensor kinase in Escherichia coli.J Bacteriol. 2004 Apr;186(7):2085-90. doi: 10.1128/JB.186.7.2085-2090.2004. J Bacteriol. 2004. PMID: 15028693 Free PMC article.
-
Poly(3-hydroxybutyrate) synthesis by recombinant Escherichia coli arcA mutants in microaerobiosis.Appl Environ Microbiol. 2006 Apr;72(4):2614-20. doi: 10.1128/AEM.72.4.2614-2620.2006. Appl Environ Microbiol. 2006. PMID: 16597965 Free PMC article.
-
The ArcB kinase sensor participates in the phagocyte-mediated stress response in Salmonella Typhimurium.Front Microbiol. 2025 Feb 11;16:1541797. doi: 10.3389/fmicb.2025.1541797. eCollection 2025. Front Microbiol. 2025. PMID: 40008041 Free PMC article.
References
-
- Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37:111–123. - PubMed
-
- Cotter P A, Melville S B, Albrecht J A, Gunsalus R P. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol Microbiol. 1997;25:605–615. - PubMed
-
- Fu H-A, Iuchi S, Lin E C C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991;226:209–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

