An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine
- PMID: 10851019
- PMCID: PMC2175113
- DOI: 10.1083/jcb.149.6.1215
An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine
Abstract
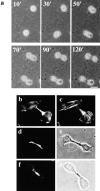
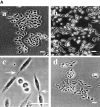
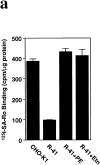
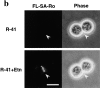
Phosphatidylethanolamine (PE) is a major membrane phospholipid that is mainly localized in the inner leaflet of the plasma membrane. We previously demonstrated that PE was exposed on the cell surface of the cleavage furrow during cytokinesis. Immobilization of cell surface PE by a PE-binding peptide inhibited disassembly of the contractile ring components, including myosin II and radixin, resulting in formation of a long cytoplasmic bridge between the daughter cells. This blockade of contractile ring disassembly was reversed by removal of the surface-bound peptide, suggesting that the PE exposure plays a crucial role in cytokinesis. To further examine the role of PE in cytokinesis, we established a mutant cell line with a specific decrease in the cellular PE level. On the culture condition in which the cell surface PE level was significantly reduced, the mutant ceased cell growth in cytokinesis, and the contractile ring remained in the cleavage furrow. Addition of PE or ethanolamine, a precursor of PE synthesis, restored the cell surface PE on the cleavage furrow and normal cytokinesis. These findings provide the first evidence that PE is required for completion of cytokinesis in mammalian cells, and suggest that redistribution of PE on the cleavage furrow may contribute to regulation of contractile ring disassembly.
Figures





References
-
- Aoki Y., Uenaka T., Aoki J., Umeda M., Inoue K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J. Biochem. 1994;116:291–297. - PubMed
-
- Bazzi M.D., Youakin M.A., Nelsestuen G.L. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry. 1992;31:1125–1134. - PubMed
-
- Bi E., Luthenhaus J. FtsZ ring structure associated with division in Escherichia coli . Nature. 1991;354:161–164. - PubMed
-
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed

