Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts
- PMID: 10851026
- PMCID: PMC2175120
- DOI: 10.1083/jcb.149.6.1297
Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts
Abstract
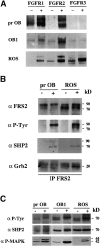
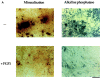
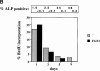
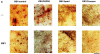
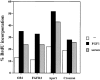
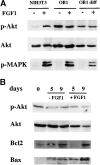
Fibroblast growth factors (FGF) play a critical role in bone growth and development affecting both chondrogenesis and osteogenesis. During the process of intramembranous ossification, which leads to the formation of the flat bones of the skull, unregulated FGF signaling can produce premature suture closure or craniosynostosis and other craniofacial deformities. Indeed, many human craniosynostosis disorders have been linked to activating mutations in FGF receptors (FGFR) 1 and 2, but the precise effects of FGF on the proliferation, maturation and differentiation of the target osteoblastic cells are still unclear. In this report, we studied the effects of FGF treatment on primary murine calvarial osteoblast, and on OB1, a newly established osteoblastic cell line. We show that FGF signaling has a dual effect on osteoblast proliferation and differentiation. FGFs activate the endogenous FGFRs leading to the formation of a Grb2/FRS2/Shp2 complex and activation of MAP kinase. However, immature osteoblasts respond to FGF treatment with increased proliferation, whereas in differentiating cells FGF does not induce DNA synthesis but causes apoptosis. When either primary or OB1 osteoblasts are induced to differentiate, FGF signaling inhibits expression of alkaline phosphatase, and blocks mineralization. To study the effect of craniosynostosis-linked mutations in osteoblasts, we introduced FGFR2 carrying either the C342Y (Crouzon syndrome) or the S252W (Apert syndrome) mutation in OB1 cells. Both mutations inhibited differentiation, while dramatically inducing apoptosis. Furthermore, we could also show that overexpression of FGF2 in transgenic mice leads to increased apoptosis in their calvaria. These data provide the first biochemical analysis of FGF signaling in osteoblasts, and show that FGF can act as a cell death inducer with distinct effects in proliferating and differentiating osteoblasts.
Figures





References
-
- Anderson J., Burns H.D., Enriquez-Harris P., Wilkie A.O.M., Heath J.K. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Human Mol. Genet. 1998;7:1475–1483. - PubMed
-
- Bellosta P., Zhang Q., Goff S.P., Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–2397. - PubMed
-
- Burke D., Wilkes D., Blundell T.L., Malcolm S. Fibroblast growth factor receptorslessons from the genes. Trends Biochem. Sci. 1998;23:259–262. - PubMed
-
- Coffin J.D., Florkiewicz R.Z., Neumann J., Mort-Hopkins T., Dorn G.W., II, Lightfoot P., German R., Howles P.N., Kier A., O'Toole B.A. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol. Biol. Cell. 1995;6:1861–1873. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

