The hydrogenase cytochrome b heme ligands of Azotobacter vinelandii are required for full H(2) oxidation capability
- PMID: 10852874
- PMCID: PMC101916
- DOI: 10.1128/JB.182.12.3429-3436.2000
The hydrogenase cytochrome b heme ligands of Azotobacter vinelandii are required for full H(2) oxidation capability
Abstract
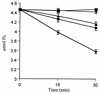
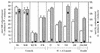
The hydrogenase in Azotobacter vinelandii, like other membrane-bound [NiFe] hydrogenases, consists of a catalytic heterodimer and an integral membrane cytochrome b. The histidines ligating the hemes in this cytochrome b were identified by H(2) oxidation properties of altered proteins produced by site-directed mutagenesis. Four fully conserved and four partially conserved histidines in HoxZ were substituted with alanine or tyrosine. The roles of these histidines in HoxZ heme binding and hydrogenase were characterized by O(2)-dependent H(2) oxidation and H(2)-dependent methylene blue reduction in vivo. Mutants H33A/Y (H33 replaced by A or Y), H74A/Y, H194A, H208A/Y, and H194,208A lost O(2)-dependent H(2) oxidation activity, H194Y and H136A had partial activity, and H97Y,H98A and H191A had full activity. These results suggest that the fully conserved histidines 33, 74, 194, and 208 are ligands to the hemes, tyrosine can serve as an alternate ligand in position 194, and H136 plays a role in H(2) oxidation. In mutant H194A/Y, imidazole (Imd) rescued H(2) oxidation activity in intact cells, which suggests that Imd acts as an exogenous ligand. The heterodimer activity, quantitatively determined as H(2)-dependent methylene blue reduction, indicated that the heterodimers of all mutants were catalytically active. H33A/Y had wild-type levels of methylene blue reduction, but the other HoxZ ligand mutants had significantly less than wild-type levels. Imd reconstituted full methylene blue reduction activity in mutants H194A/Y and H208A/Y and partial activity in H194,208A. These results indicate that structural and functional integrity of HoxZ is required for physiologically relevant H(2) oxidation, and structural integrity of HoxZ is necessary for full heterodimer-catalyzed H(2) oxidation.
Figures

References
-
- Arp D J. Hydrogen-oxidizing bacteria: methods used in their investigation. In: Linskens H R, Jackson J F, editors. Gases in plant and microbial cells. New York, N.Y: Springer-Verlag; 1989. pp. 257–274.
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1998.
-
- Barrick D. Replacement of the proximal ligand of sperm whale myoglobin with free imidazole in the mutant his-93→gly. Biochemistry. 1994;33:6546–6554. - PubMed
-
- Bernhard M, Benelli B, Hochkoeppler A, Zannoni D, Friedrich B. Functional and structural role of the cytochrome b subunit of the membrane-bound hydrogenase complex of Alcaligenes eutrophus H16. Eur J Biochem. 1997;248:179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

