The dual-specificity protein phosphatase Yvh1p regulates sporulation, growth, and glycogen accumulation independently of catalytic activity in Saccharomyces cerevisiae via the cyclic AMP-dependent protein kinase cascade
- PMID: 10852885
- PMCID: PMC101947
- DOI: 10.1128/JB.182.12.3517-3528.2000
The dual-specificity protein phosphatase Yvh1p regulates sporulation, growth, and glycogen accumulation independently of catalytic activity in Saccharomyces cerevisiae via the cyclic AMP-dependent protein kinase cascade
Abstract
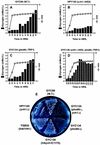
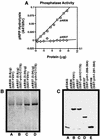
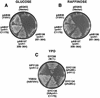
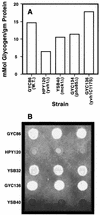
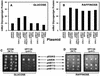
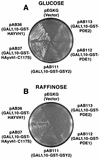
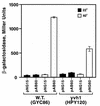
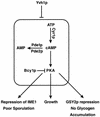
Yvh1p, a dual-specific protein phosphatase induced specifically by nitrogen starvation, regulates cell growth as well as initiation and completion of sporulation. We demonstrate that yvh1 disruption mutants are also unable to accumulate glycogen in stationary phase. A catalytically inactive variant of yvh1 (C117S) and a DNA fragment encoding only the Yvh1p C-terminal 159 amino acids (which completely lacks the phosphatase domain) complement all three phenotypes as well as the wild-type allele; no complementation occurs with a fragment encoding only the C-terminal 74 amino acids. These observations argue that phosphatase activity is not required for the Yvh1p functions we measured. Mutations which decrease endogenous cyclic AMP (cAMP) levels partially suppress the sporulation and glycogen accumulation defects. In addition, reporter gene expression supported by a DRR2 promoter fragment, containing two stress response elements known to respond to cAMP-protein kinase A, decreases in a yvh1 disruption mutant. Therefore, our results identify three cellular processes that both require Yvh1p and respond to alterations in cAMP, and they lead us to suggest that Yvh1p may be a participant in and/or a contributor to regulation of the cAMP-dependent protein kinase cascade. The fact that decreasing the levels of cAMP alleviates the need for Yvh1p function supports this suggestion.
Figures


References
-
- Botstein D, Falco S C, Stewart S E, Brennan M, Scherer S, Stinchomb D T, Struhl K, Davis R W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

