Identification of iron-responsive, differential gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803 with a customized amplification library
- PMID: 10852887
- PMCID: PMC101951
- DOI: 10.1128/JB.182.12.3536-3543.2000
Identification of iron-responsive, differential gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803 with a customized amplification library
Abstract
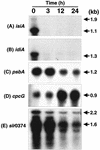
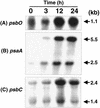
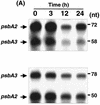
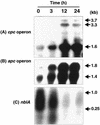
We describe the use of a method called differential expression using customized amplification library (DECAL) to study the global changes in gene expression in iron-deficient versus iron-reconstituting cells of Synechocystis sp. strain PCC 6803. We identified a number of genes, such as isiA, idiA, psbA, cpcG, and slr0374, whose expression either increased or decreased in response to iron availability. Further analysis led to the identification of additional genes related to those identified by DECAL (e.g., psbC, psbO, psaA, apcABC, cpcBAC1C2D, and nblA) that were differentially regulated by iron availability. Expression of cpcG, psbC, psbO, psaA, apcABC, and cpcBAC1C2D increased, whereas that of isiA, idiA, nblA, psbA, and slr0374 decreased, in iron-reconstituting cells. S1 nuclease protection studies showed that increased transcript levels of psbA in iron-deficient cells was due to the increased expression of both psbA2 and psbA3 genes, although the steady-state level of psbA2 remained higher than that of psbA3.
Figures

References
-
- Alland D, Kramnik I, Weisbrod T R, Otsubo L, Cerny R, Miller L P, Jacobs W R J, Bloom B R. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:13227–13232. - PMC - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1999.
-
- Behrenfeld M J, Kolber Z S. Widespread iron limitation of phytoplankton in the South Pacific Ocean. Science. 1999;283:840–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

