Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture
- PMID: 10852895
- PMCID: PMC101973
- DOI: 10.1128/JB.182.12.3593-3596.2000
Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture
Abstract
Although exopolysaccharides (EPSs) are a large component of bacterial biofilms, their contribution to biofilm structure and function has been examined for only a few organisms. In each of these cases EPS has been shown to be required for cellular attachment to abiotic surfaces. Here, we undertook a genetic approach to examine the potential role of colanic acid, an EPS of Escherichia coli K-12, in biofilm formation. Strains either proficient or deficient in colanic acid production were grown and allowed to adhere to abiotic surfaces and were then examined both macroscopically and microscopically. Surprisingly, we found that colanic acid production is not required for surface attachment. Rather, colanic acid is critical for the formation of the complex three-dimensional structure and depth of E. coli biofilms.
Figures
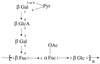


References
-
- Carr J H, Anderson R L, Favero M S. Comparison of chemical dehydration and critical point drying for the stabilization and visualization of aging biofilm present on interior surfaces of PVC distribution pipe. J Appl Bacteriol. 1996;80:225–232. - PubMed
-
- Costerton J W. Overview of microbial biofilms. J Ind Microbiol. 1995;15:137–140. - PubMed
-
- Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. - PubMed
-
- Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

