A shaker-like K(+) channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis
- PMID: 10852932
- PMCID: PMC149088
- DOI: 10.1105/tpc.12.6.837
A shaker-like K(+) channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis
Abstract
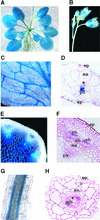
RNA gel blot and reverse transcription-polymerase chain reaction experiments were used to identify a single K(+) channel gene in Arabidopsis as expressed throughout the plant. Use of the beta-glucuronidase reporter gene revealed expression of this gene, AKT2/AKT3, in both source and sink phloem tissues. The AKT2/AKT3 gene corresponds to two previously identified cDNAs, AKT2 (reconstructed at its 5' end) and AKT3, the open reading frame of the latter being shorter at its 5' end than that of the former. Rapid amplification of cDNA ends with polymerase chain reaction and site-directed mutagenesis was performed to identify the initiation codon for AKT2 translation. All of the data are consistent with the hypothesis that the encoded polypeptide corresponds to the longest open reading frame previously identified (AKT2). Electrophysiological characterization (macroscopic and single-channel currents) of AKT2 in both Xenopus oocytes and COS cells revealed a unique gating mode and sensitivity to pH (weak inward rectification, inhibition, and increased rectification upon internal or external acidification), suggesting that AKT2 has enough functional plasticity to perform different functions in phloem tissue of source and sink organs. The plant stress hormone abscisic acid was shown to increase the amount of AKT2 transcript, suggesting a role for the AKT2 in the plant response to drought.
Figures


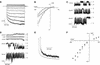


Similar articles
-
Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA.Plant Cell. 2002 May;14(5):1133-46. doi: 10.1105/tpc.000943. Plant Cell. 2002. PMID: 12034902 Free PMC article.
-
In planta AKT2 subunits constitute a pH- and Ca2+-sensitive inward rectifying K+ channel.Planta. 2007 Apr;225(5):1179-91. doi: 10.1007/s00425-006-0428-4. Epub 2006 Dec 5. Planta. 2007. PMID: 17146665
-
Outer pore residues control the H(+) and K(+) sensitivity of the Arabidopsis potassium channel AKT3.Plant Cell. 2002 Aug;14(8):1859-68. doi: 10.1105/tpc.003244. Plant Cell. 2002. PMID: 12172027 Free PMC article.
-
Isolation of AtSUC2 promoter-GFP-marked companion cells for patch-clamp studies and expression profiling.Plant J. 2003 Dec;36(6):931-45. doi: 10.1046/j.1365-313x.2003.01931.x. Plant J. 2003. PMID: 14675456
-
Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation.Plant J. 2005 Dec;44(5):783-97. doi: 10.1111/j.1365-313X.2005.02566.x. Plant J. 2005. PMID: 16297070
Cited by
-
Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA.Plant Cell. 2002 May;14(5):1133-46. doi: 10.1105/tpc.000943. Plant Cell. 2002. PMID: 12034902 Free PMC article.
-
The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants.Plants (Basel). 2023 Jan 3;12(1):204. doi: 10.3390/plants12010204. Plants (Basel). 2023. PMID: 36616332 Free PMC article.
-
Systemic nature of drought-tolerance in common bean.Plant Signal Behav. 2008 Sep;3(9):663-6. doi: 10.4161/psb.3.9.5776. Plant Signal Behav. 2008. PMID: 19704819 Free PMC article.
-
Non-autonomous stomatal control by pavement cell turgor via the K+ channel subunit AtKC1.Plant Cell. 2022 Apr 26;34(5):2019-2037. doi: 10.1093/plcell/koac038. Plant Cell. 2022. PMID: 35157082 Free PMC article.
-
Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):864-9. doi: 10.1073/pnas.1009777108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187374 Free PMC article.
References
-
- Barhanin, J., Lesage, F., Guillemare, E., Fink, M., Lazdunski, M., and Romey, G. (1996). KvLQT1 and IsK (MinK) proteins associate to form the IKs cardiac potassium current. Nature 384, 78–83. - PubMed
-
- Cao, Y., Ward, J.M., Kelly, W.B., Ichida, A.M., Gaber, R.F., Anderson, J.A., Uozumi, N., Schroeder, J.I., and Crawford, N.M. (1995). Multiple genes, tissue specificity, and expression-dependent modulation contribute to functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 109, 1093–1106. - PMC - PubMed
-
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

