v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation
- PMID: 10852971
- PMCID: PMC16538
- DOI: 10.1073/pnas.140210297
v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation
Abstract
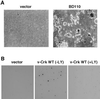
v-Crk induces cellular tyrosine phosphorylation and transformation of chicken embryo fibroblasts (CEF). We studied the molecular mechanism of the v-Crk-induced transformation. Experiments with Src homology (SH)2 and SH3 domain mutants revealed that the induction of tyrosine phosphorylation of cellular proteins requires only the SH2 domain, but both the SH2 and SH3 domains are required for complete transformation. Analysis of three well defined signaling pathways, the mitogen-activated protein kinase (MAPK) pathway, the Jun N-terminal kinase (JNK) pathway, and the phosphoinositide 3-kinase (PI3K)/AKT pathway, demonstrated that only the PI3K/AKT pathway is constitutively activated in v-Crk-transformed CEF. Both the SH2 and SH3 domains are required for this activation of the PI3K/AKT pathway in CEF. We also found that the colony formation of CEF is strongly induced by a constitutively active PI3K mutant, and that a PI3K inhibitor, LY294002, suppresses the v-Crk-induced transformation. These results strongly suggest that constitutive activation of the PI3K/AKT pathway plays an essential role in v-Crk-induced transformation of CEF.
Figures



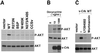
References
-
- Mayer B J, Hamaguchi M, Hanafusa H. Nature (London) 1988;332:272–275. - PubMed
-
- Reichman C T, Mayer B J, Keshav S, Hanafusa H. Cell Growth Differ. 1992;3:451–460. - PubMed
-
- Pawson T, Gish G D. Cell. 1992;71:359–362. - PubMed
-
- Birge R B, Knudsen B S, Besser D, Hanafusa H. Genes Cells. 1996;1:595–613. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

