Induction of apoptosis in rat cardiocytes by A3 adenosine receptor activation and its suppression by isoproterenol
- PMID: 10854059
- PMCID: PMC10792615
- DOI: 10.1006/excr.2000.4882
Induction of apoptosis in rat cardiocytes by A3 adenosine receptor activation and its suppression by isoproterenol
Abstract
The purpose of the present study was to investigate the mechanisms involved in the induction of apoptosis in newborn cultured cardiomyocytes by activation of adenosine (ADO) A3 receptors and to examine the protective effects of beta-adrenoceptors. The selective agonist for A3 ADO receptors Cl-IB-MECA (2-chloro-N6-iodobenzyl-5-N-methylcarboxamidoadenosine) and the antagonist MRS1523 (5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpy rid ine-5-carboxylate) were used. High concentrations of the Cl-IB-MECA (> or = 10 microM) agonist induced morphological modifications of myogenic cells, such as rounding and retraction of cell body and dissolution of contractile filaments, followed by apoptotic death. In addition, Cl-IB-MECA caused a sustained and reversible increase in [Ca2+]i, which was prevented by the selective antagonist MRS1523. Furthermore, MRS1523 protected the cardiocytes if briefly exposed to Cl-IB-MECA and partially protected from prolonged (48 h) agonist exposure. Apoptosis induced by Cl-IB-MECA was not redox-dependent, since the mitochondrial membrane potential remained constant until the terminal stage of cell death. Cl-IB-MECA activated caspase-3 protease in a concentration-dependent manner after 7 h of treatment and more effectively after 18 h of exposure. Bcl-2 protein was readily detected in control cells, and its expression was significantly decreased after 24 and 48 h of treatment with Cl-IB-MECA. Beta-adrenergic stimulation antagonized the pro-apoptotic effects of Cl-IB-MECA, probably through a cAMP/protein kinase A-independent mechanism, since addition of dibutyryl-cAMP did not abolish the apoptosis induced by Cl-IB-MECA. Incubation of cultured myocytes with isoproterenol (5 microM) for 3 or 24 h almost completely abolished the increase in [Ca2+]i. Prolonged incubation of cardiomyocytes with isoproterenol and Cl-IB-MECA did not induce apoptosis. Our data suggest that the apoptosis-inducing signal from activation of adenosine A3 receptors (or counteracting beta-adrenergic signal) leads to the activation of the G-protein-coupled enzymes and downstream pathways to a self-amplifying cascade. Expression of different genes within this cascade is responsible for orchestrating either cardiomyocyte apoptosis or its protection.
Figures
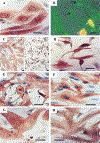

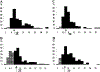
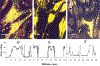

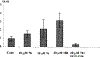
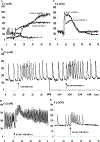


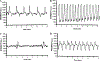
References
-
- Giannella E, Mochmann HC, and Levi R (1997). Ischemic preconditioning prevents the impairment of hypoxic coronary vasodilatation caused by ischemia/reperfusion: Role of adenosine A1/A3 and bradykinin B2 receptor activation. Circ. Res 81, 415–422. - PubMed
-
- Fozard JR, Pfannkuche HJ, and Schuurman HJ (1996). Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol 298, 293–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

