Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction
- PMID: 10854210
- PMCID: PMC1850078
- DOI: 10.1016/S0002-9440(10)65060-2
Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction
Abstract
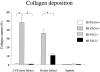
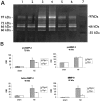
The plasminogen system plays an important role in the proteolytic degradation of extracellular matrices during wound healing. In the present study we investigated the impact of the plasminogen system on cardiac wound healing and function after myocardial infarction. Myocardial infarction was induced in plasminogen-deficient mice (Plg-/-) and in wild-type controls (Plg+/+). Structural analysis 1, 2, and 5 weeks after infarction revealed that infarct healing was virtually abolished in Plg-/- mice, indicating that the plasminogen system is required for the repair process of the heart after infarction. In the absence of plasminogen, inflammatory cells did not migrate into the infarcted myocardium. Necrotic cardiomyocytes were not removed and the formation of granulation tissue and fibrous tissue did not occur. In these non-healing infarcted hearts, LV dilatation was not altered. In addition, gelatinolytic activity of MMP-2 and MMP-9 was depressed in the Plg-/- infarcted hearts, suggesting that the plasmin effect on infarct healing may be mediated by MMPs. Surprisingly, cardiac function was only attenuated to a rather small extent in the Plg-/- infarcted mice when compared to the wild-types. This study provides direct prove that plasmin-mediated proteolysis plays a central role in cardiac wound healing after myocardial infarction in mice.
Figures






References
-
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JPM, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P: Inhibition of plasminogen activators or matrix metalloproteinases prevent cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 1999, 10:1135-1142 - PubMed
-
- Matrisian LM: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990, 6:121-125 - PubMed
-
- Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D: Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 1997, 17:439-444 - PubMed
-
- Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, Van den Oord JJ, Collen D, Mulligan RC: Physiological consequences of loss of plasminogen activator gene function in mice. Nature 1994, 368:419-424 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

