Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus
- PMID: 10854218
- PMCID: PMC1850091
- DOI: 10.1016/S0002-9440(10)65068-7
Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus
Abstract
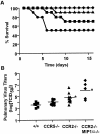
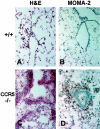
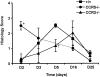
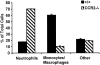
The immune response to influenza A virus is characterized by an influx of both macrophages and T lymphocytes into the lungs of the infected host, accompanied by induced expression of a number of CC chemokines. CC chemokine receptors CCR5 and CCR2 are both expressed on activated macrophages and T cells. We examined how the absence of these chemokine receptors would affect pulmonary chemokine expression and induced leukocyte recruitment by infecting CCR5-deficient mice and CCR2-deficient mice with a mouse-adapted strain of influenza A virus. CCR5(-/-) mice displayed increased mortality rates associated with acute, severe pneumonitis, whereas CCR2(-/-) mice were protected from the early pathological manifestations of influenza because of defective macrophage recruitment. This delay in macrophage accumulation in CCR2(-/-) mice caused a subsequent delay in T cell migration, which correlated with high pulmonary viral titers at early time points. Infected CCR5(-/-) mice and CCR2(-/-) mice both exhibited increased expression of the gene for MCP-1, the major ligand for CCR2(-/-) and a key regulator of induced macrophage migration. These studies illustrate the very different roles that CCR5 and CCR2 play in the macrophage response to influenza infection and demonstrate how defects in macrophage recruitment affect the normal development of the cell-mediated immune response.
Figures

References
-
- Cook DN: The role of MIP-1α in inflammation and hematopoiesis. J Leukoc Biol 1996, 59:61-66 - PubMed
-
- Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 - PubMed
-
- Ward SG, Bacon K, Westwick J: Chemokines and T lymphocytes: more than an attraction. Immunity 1998, 9:1-11 - PubMed
-
- Zlotnik A, Morales J, Hedrick JA: Recent advances in chemokines and chemokine receptors. Crit Rev Immunol 1999, 19:1-47 - PubMed
-
- Devalaraja MN, Richmond A: Multiple chemotactic factors: fine control or redundancy? Trends Pharm Sci 1999, 20:151-156 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

