Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver
- PMID: 10854224
- PMCID: PMC1850065
- DOI: 10.1016/S0002-9440(10)65074-2
Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver
Abstract
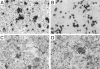
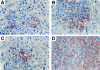
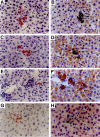
To identify cells that have the ability to proliferate and differentiate into all epithelial components of the liver lobule, we isolated fetal liver epithelial cells (FLEC) from ED 14 Fischer (F) 344 rats and transplanted these cells in conjunction with two-thirds partial hepatectomy into the liver of normal and retrorsine (Rs) treated syngeneic dipeptidyl peptidase IV mutant (DPPIV(-)) F344 rats. Using dual label immunohistochemistry/in situ hybridization, three subpopulations of FLEC were identified: cells expressing both alpha-fetoprotein (AFP) and albumin, but not CK-19; cells expressing CK-19, but not AFP or albumin, and cells expressing AFP, albumin, and cytokeratins-19 (CK-19). Proliferation, differentiation, and expansion of transplanted FLEC differed significantly in the two models. In normal liver, 1 to 2 weeks after transplantation, mainly cells with a single phenotype, hepatocytic (expressing AFP and albumin) or bile ductular (expressing only CK-19), had proliferated. In Rs-treated rats, in which the proliferative capacity of endogenous hepatocytes is impaired, transplanted cells showed mainly a dual phenotype (expressing both AFP/albumin and CK-19). One month after transplantation, DPPIV(+) FLEC engrafted into the parenchyma exhibited an hepatocytic phenotype and generated new hepatic cord structures. FLEC, localized in the vicinity of bile ducts, exhibited a biliary epithelial phenotype and formed new bile duct structures or were incorporated into pre-existing bile ducts. In the absence of a proliferative stimulus, ED 14 FLEC did not proliferate or differentiate. Our results demonstrate that 14-day fetal liver contains lineage committed (unipotential) and uncommitted (bipotential) progenitor cells exerting different repopulating capacities, which are affected by the proliferative status of the recipient liver and the host site within the liver where the transplanted cells become engrafted. These findings have important implications in future studies directed toward liver repopulation and ex vivo gene therapy.
Figures







References
-
- DuBois AM: The embryonic liver. The Liver. Edited by Rouiller CH. New York, Academic Press, 1963
-
- Wilson JW, Groat CS, Leduc EH: Histogenesis of the liver. Ann NY Acad Sci 1963, 111:8-22 - PubMed
-
- Rugh R: The Mouse: Its Reproduction and Development. 1968. Oxford University Press, New York
-
- Le Douarin NM: An experimental analysis of liver development. Med Biol 1975, 53:427-455 - PubMed
-
- Houssaint E: Differentiation of mouse hepatic primordium: an analysis of tissue interactions in hepatocyte differentiation. Cell Growth Differ 1980, 9:269-279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

