Parallel genotyping of human SNPs using generic high-density oligonucleotide tag arrays
- PMID: 10854416
- PMCID: PMC310915
- DOI: 10.1101/gr.10.6.853
Parallel genotyping of human SNPs using generic high-density oligonucleotide tag arrays
Abstract
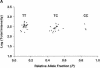
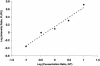
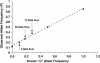
Large scale human genetic studies require technologies for generating millions of genotypes with relative ease but also at a reasonable cost and with high accuracy. We describe a highly parallel method for genotyping single nucleotide polymorphisms (SNPs), using generic high-density oligonucleotide arrays that contain thousands of preselected 20-mer oligonucleotide tags. First, marker-specific primers are used in PCR amplifications of genomic regions containing SNPs. Second, the amplification products are used as templates in single base extension (SBE) reactions using chimeric primers with 3' complementarity to the specific SNP loci and 5' complementarity to specific probes, or tags, synthesized on the array. The SBE primers, terminating one base before the polymorphic site, are extended in the presence of labeled dideoxy NTPs, using a different label for each of the two SNP alleles, and hybridized to the tag array. Third, genotypes are deduced from the fluorescence intensity ratio of the two colors. This approach takes advantage of multiplexed sample preparation, hybridization, and analysis at each stage. We illustrate and test this method by genotyping 44 individuals for 142 human SNPs identified previously in 62 candidate hypertension genes. Because the hybridization results are quantitative, this method can also be used for allele-frequency estimation in pooled DNA samples.
Figures




References
-
- Cargill M, Altshuler D, Ireland J, Sklar P, Ardlie K, Patil N, Lane CR, Lim EP, Kalayanaraman N, Nemesh J, et al. Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nature Genet. 1999;22:231–238. - PubMed
-
- Chakravarti A. Population genetics–making sense out of sequence. Nature Genet. 1999;21:56–60. - PubMed
-
- Chee M, Yang R, Hubbell E, Berno A, Huang XC, Stern D, Winkler J, Lockhart DJ, Morris MS, Fodor SP. Accessing genetic information with high-density DNA arrays. Science. 1996;274:610–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
