Phosphorylation status of the SCR homeodomain determines its functional activity: essential role for protein phosphatase 2A,B'
- PMID: 10856239
- PMCID: PMC203353
- DOI: 10.1093/emboj/19.12.2946
Phosphorylation status of the SCR homeodomain determines its functional activity: essential role for protein phosphatase 2A,B'
Abstract
Sex combs reduced (SCR) is a Drosophila Hox protein that determines the identity of the labial and prothoracic segments. In search of factors that might associate with SCR to control its activity and/or specificity, we performed a yeast two-hybrid screen. A Drosophila homologue of the regulatory subunit (B'/PR61) of serine-threonine protein phosphatase 2A (dPP2A,B') specifically interacted with the SCR homeodomain. The N-terminal arm within the SCR homeodomain was shown to be a target of phosphorylation/dephosphorylation by cAMP-dependent protein kinase A and protein phosphatase 2A, respectively. In vivo analyses revealed that mutant forms of SCR mimicking constitutively dephosphorylated or phosphorylated states of the homeodomain were active or inactive, respectively. Inactivity of the phosphorylated mimic form was attributed to impaired DNA binding. Specific ablation of dPP2A,B' gene activity by double-stranded RNA-mediated genetic interference resulted in embryos without salivary glands, an SCR null phenotype. Our data demonstrate an essential role for Drosophila PP2A,B' in positively modulating SCR function.
Figures


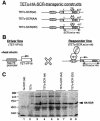
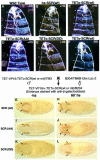
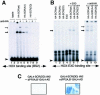

References
-
- Andrew D.J., Baig,A., Bhanot,P., Smolik,S.M. and Henderson,K.D. (1997) The Drosophila dCREB-A gene is required for dorsal/ventral patterning of the larval cuticle. Development, 124, 181–193. - PubMed
-
- Bartel P.L. and Fields,S. (1995) Analyzing protein–protein interactions using two-hybrid system. Methods Enzymol., 254, 241–263. - PubMed
-
- Bello B., Resendez-Perez,D. and Gehring,W.J. (1998) Spatial and temporal targeting of gene expression in Drosophila by means of a tetracycline-dependent transactivator system. Development, 125, 2193–2202. - PubMed
-
- Bourbon H.M., Martin-Blanco,E., Rosen,D. and Kornberg,T.B. (1995) Phosphorylation of the Drosophila engrailed protein at a site outside its homeodomain enhances DNA binding. J. Biol. Chem., 270, 11130–11139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

