The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators
- PMID: 10856251
- PMCID: PMC203345
- DOI: 10.1093/emboj/19.12.3080
The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators
Abstract
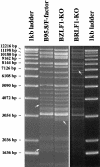
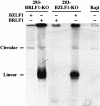
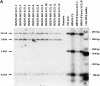
The propagation of herpesviruses has long been viewed as a temporally regulated sequential process that results from the consecutive expression of specific viral transactivators. As a key step in this process, lytic viral DNA replication is considered as a checkpoint that controls the expression of the late structural viral genes. In a novel genetic approach, we show that both hypotheses do not hold true for the Epstein-Barr virus (EBV). The study of viral mutants of EBV in which the early genes BZLF1 and BRLF1 are deleted allowed a precise assignment of the function of these proteins. Both transactivators were absolutely essential for viral DNA replication. Both BZLF1 and BRLF1 were required for full expression of the EBV proteins expressed during the lytic program, although the respective influence of these molecules on the expression of various viral target genes varied greatly. In replication-defective viral mutants, neither early gene expression nor DNA replication was a prerequisite for late gene expression. This work shows that BRLF1 and BZLF1 harbor distinct but complementary functions that influence all stages of viral production.
Figures






References
-
- Baer R. et al. (1984) DNA sequence and expression of the B95-8 Epstein–Barr virus genome. Nature, 310, 207–211. - PubMed
-
- Cherepanov P.P. and Wackernagel,W. (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene, 158, 9–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

