Two-dimensional NMR spectroscopy and structures of six lipid A species from Rhizobium etli CE3. Detection of an acyloxyacyl residue in each component and origin of the aminogluconate moiety
- PMID: 10856304
- PMCID: PMC2570648
- DOI: 10.1074/jbc.M004009200
Two-dimensional NMR spectroscopy and structures of six lipid A species from Rhizobium etli CE3. Detection of an acyloxyacyl residue in each component and origin of the aminogluconate moiety
Abstract
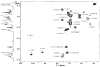
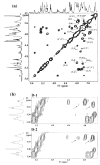
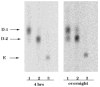
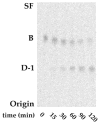
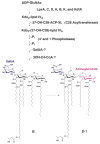
The chemical structures of six lipid A species (A, B, C, D-1, D-2, and E) purified from Rhizobium etli CE3 were investigated by one- and two-dimensional NMR spectroscopy. The R. etli lipid A subtypes each contain an unusual acyloxyacyl residue at position 2' as part of a conserved distal glucosamine moiety but differ in their proximal units. All R. etli lipid A species lack phosphate groups. However, they are derivatized with an alpha-linked galacturonic acid group at position 4', as shown by nuclear Overhauser effect spectroscopy. Component B, which had been not been reported in previous studies, features a beta, 1'-6 linked disaccharide of glucosamine acylated at positions 2, 3, 2', and 3' in a pattern that is typical of lipid A found in other Gram-negative bacteria. D-1 contains an acylated aminogluconate unit in place of the proximal glucosamine residue of B. C and E lack ester-linked beta-hydroxyacyl chains at position 3, as judged by their H-3 chemical shifts, and may be synthesized from B and D-1, respectively, by the R. etli 3-O-deacylase. D-2 is an isomer of D-1 that forms nonenzymatically by acyl chain migration. A may be an elimination product derived from D-1 during hydrolysis at 100 degrees C (pH 4.5), a step needed to release lipid A from lipopolysaccharide. Based on these findings, we propose a biosynthetic scheme for R. etli lipid A in which B is generated first by a variation of the E. coli pathway. The aminogluconate unit of D-1 could then be made from B by enzymatic oxidation of the proximal glucosamine. As predicted by our hypothesis, enzyme(s) can be demonstrated in extracts of R. etli that convert (14)C-labeled B to D-1.
Figures







References
-
- Long SR, Staskawicz BJ. Cell. 1993;73:921–935. - PubMed
-
- Spaink HP, Kondorosi A, Hooykaas PJJ. The Rhizobiaceae: Molecular Biology of Model Plant-associated Bacteria. Kluwer; Dordrecht, The Netherlands: 1998.
-
- Kannenberg EL, Brewin NJ. Trends Microbiol. 1994;2:277–283. - PubMed
-
- Carlson RW, Bhat UR, Reuhs B. In: Plant Bio/Technology and Development. Gresshoff PM, editor. CRC Press; Boca Raton, FL: 1992. pp. 33–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

