Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage
- PMID: 10858191
- PMCID: PMC101655
- DOI: 10.1128/IAI.68.7.3830-3839.2000
Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage
Abstract
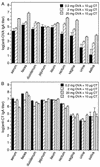
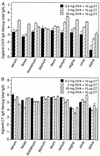
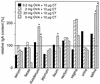
Monitoring specific secretory immunoglobulin A (IgA) responses in the intestines after mucosal immunization or infection is impeded by the fact that sampling of small intestinal secretions requires invasive methods not feasible for routine diagnostics. Since IgA plasma cells generated after intragastric immunization are known to populate remote mucosal sites as well, secretory IgA responses at other mucosal surfaces may correlate to those in the intestines and could serve as proxy measures for IgA secretion in the gut. To evaluate the practicability of this approach, mice were immunized intragastrically with 0.2, 2, and 20 mg of ovalbumin plus 10 microg of cholera toxin, and the antigen-specific local secretory IgA responses in duodenal, ileal, jejunal, rectal, and vaginal secretions, saliva, urine, and feces, as well as serum IgG and IgA responses were analyzed by enzyme-linked immunosorbent assay. Correlation analysis revealed significant relationships between serum IgG and IgA, urinary IgA, salivary IgA, and secretory IgA in duodenal, jejunal, ileal, and rectal secretions for the 0.2-mg but not for the 20-mg ovalbumin dose. Fecal samples were poor predictors for intestinal antiovalbumin IgA responses, and no correlations could be established for cholera toxin, neither between local anti-cholera toxin levels nor to the antiovalbumin responses. Thus, specific IgA in serum, saliva, or urine can serve as a predictor of the release of specific IgA at intestinal surfaces after intragastric immunization, but the lack of correlations for high ovalbumin doses and for cholera toxin indicates a strong dependency on antigen type and dosage for these relationships.
Figures
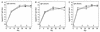


References
-
- Åhren C, Andersson K, Wiklund G, Wennerås C, Svennerholm A-M. Optimization of the intestinal lavage procedure for determination of intestinal immune responses. Vaccine. 1995;13:1754–1758. - PubMed
-
- Barton J R, O'Mahoney S, Ferguson A. Regulation of antibodies to food proteins within the common mucosal immune system: lack of correlation between antibody titers in saliva and intestinal fluid. Adv Mucosal Immunol. 1990;1990:495–496.
-
- Cancellieri V, Fara G M. Demonstration of specific IgA in human feces after immunization with live Ty21a Salmonella typhi vaccine. J Infect Dis. 1985;151:482–484. - PubMed
-
- Elson C O, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. - PubMed
-
- Elson C O, Ealding W, Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984;67:101–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

