Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection
- PMID: 10858240
- PMCID: PMC101732
- DOI: 10.1128/IAI.68.7.4225-4237.2000
Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection
Abstract
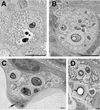
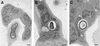
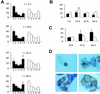
To produce chronic infection, microbial pathogens must escape host immune defenses. Infection with the human pathogenic fungus Cryptococcus neoformans is typically chronic. To understand the mechanism by which C. neoformans survives in tissue after the infection of immunocompetent hosts, we systematically studied the course of pulmonary infection in mice by electron microscopy. The macrophage was the primary phagocytic cell at all times of infection, but neutrophils also ingested yeast. Alveolar macrophages rapidly internalized yeast cells after intratracheal infection, and intracellular yeast cells were noted at all times of infection from 2 h through 28 days. However, the proportion of yeast cells in the intracellular and extracellular spaces varied with the time of infection. Early in infection, yeast cells were found predominantly in the intracellular compartment. A shift toward extracellular predominance occurred by 24 h that was accompanied by macrophage cytotoxicity and disruption. Later in infection, intracellular persistence in vivo was associated with replication, residence in a membrane-bound phagosome, polysaccharide accumulation inside cells, and cytotoxicity to macrophages, despite phagolysosomal fusion. Many phagocytic vacuoles with intracellular yeast had discontinuous membranes. Macrophage infection resulted in cells with a distinctive appearance characterized by large numbers of vacuoles filled with polysaccharide antigen. Similar results were observed in vitro using a macrophage-like cell line. Our results show that C. neoformans is a facultative intracellular pathogen in vivo. Furthermore, our observations suggest that C. neoformans occupies a unique niche among the intracellular pathogens whereby survival in phagocytic cells is accompanied by intracellular polysaccharide production.
Figures








References
-
- Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L-A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. - PMC - PubMed
-
- Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. - PubMed
-
- Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: American Society for Microbiology; 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

