Intracellular trafficking of Brucella abortus in J774 macrophages
- PMID: 10858243
- PMCID: PMC101738
- DOI: 10.1128/IAI.68.7.4255-4263.2000
Intracellular trafficking of Brucella abortus in J774 macrophages
Abstract
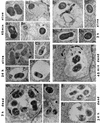
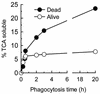
Brucella abortus is a facultative intracellular bacterium capable of surviving inside professional and nonprofessional phagocytes. The microorganism remains in membrane-bound compartments that in several cell types resemble modified endoplasmic reticulum structures. To monitor the intracellular transport of B. abortus in macrophages, the kinetics of fusion of phagosomes with preformed lysosomes labeled with colloidal gold particles was observed by electron microscopy. The results indicated that phagosomes containing live B. abortus were reluctant to fuse with lysosomes. Furthermore, newly endocytosed material was not incorporated into these phagosomes. These observations indicate that the bacteria strongly affect the normal maturation process of macrophage phagosomes. However, after overnight incubation, a significant percentage of the microorganisms were found in large phagosomes containing gold particles, resembling phagolysosomes. Most of the Brucella bacteria present in phagolysosomes were not morphologically altered, suggesting that they can also resist the harsh conditions prevalent in this compartment. About 50% colocalization of B. abortus with LysoSensor, a weak base that accumulates in acidic compartments, was observed, indicating that the B. abortus bacteria do not prevent phagosome acidification. In contrast to what has been described for HeLa cells, only a minor percentage of the microorganisms were found in compartments labeled with monodansylcadaverine, a marker for autophagosomes, and with DiOC6 (3,3'-dihexyloxacarbocyanine iodide), a marker for the endoplasmic reticulum. These results indicate that B. abortus bacteria alter phagosome maturation in macrophages. However, acidification does occur in these phagosomes, and some of them can eventually mature to phagolysosomes.
Figures
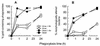



References
-
- Alvarez-Dominguez C, Roberts R, Stahl P. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J Cell Sci. 1997;110:731–743. - PubMed
-
- Beron W, Alvarez-Dominguez C, Mayorga L, Stahl P D. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 1995;5:100–104. - PubMed
-
- Clague M J, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. - PubMed
-
- Detilleux P G, Cheville N F, Deyoe B L. Pathogenesis of Brucella abortus in chicken embryos. Vet Pathol. 1988;25:138–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

