Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins
- PMID: 10858260
- PMCID: PMC101769
- DOI: 10.1128/IAI.68.7.4358-4360.2000
Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins
Abstract
Adhesion of Trichomonas vaginalis is believed to be dependent on four adhesion proteins, which are thought to bind to vaginal epithelial cells in a specific manner with a ligand-receptor type of interaction. However, the specific receptors on the host cell have not yet been identified. In this work, the ability of the T. vaginalis adhesins to bind to cells of different histologic derivations and from different species has been studied. HeLa, CHO, and Vero cell lines; erythrocytes from different species; and a prokaryote without a cell wall, Mycoplasma hominis, were employed in order to investigate the cell specificity of the T. vaginalis adhesins. We observed that the T. vaginalis adhesins are able to bind to the different cell types to the same extent, suggesting that the host and tissue specificity of T. vaginalis adhesion should not be due to specificity of the parasite adhesins. Our results suggest that the data published to date on the subject are probably artifactual and that the experiments reported in the literature are not appropriate for identification of protozoan adhesins.
Figures
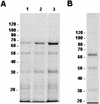
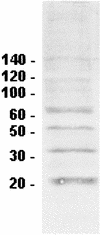
References
-
- Addis M F, Rappelli P, Cappuccinelli P, Fiori P L. Extracellular release by Trichomonas vaginalis of a NADP+ dependent malic enzyme involved in pathogenicity. Microb Pathog. 1997;23:55–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

