In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (-)-beta-D-dioxolane-guanosine and suppress resistance to 3'-azido-3'-deoxythymidine
- PMID: 10858331
- PMCID: PMC89962
- DOI: 10.1128/AAC.44.7.1783-1788.2000
In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (-)-beta-D-dioxolane-guanosine and suppress resistance to 3'-azido-3'-deoxythymidine
Abstract
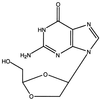
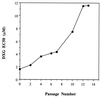
Human immunodeficiency virus type 1 (HIV-1) isolates resistant to (-)-beta-D-dioxolane-guanosine (DXG), a potent and selective nucleoside analog HIV-1 reverse transcriptase (RT) inhibitor, were selected by serial passage of HIV-1(LAI) in increasing drug concentrations (maximum concentration, 30 microM). Two independent selection experiments were performed. Viral isolates for which the DXG median effective concentrations (EC(50)s) increased 7.3- and 12.2-fold were isolated after 13 and 14 passages, respectively. Cloning and DNA sequencing of the RT region from the first resistant isolate identified a K65R mutation (AAA to AGA) in 10 of 10 clones. The role of this mutation in DXG resistance was confirmed by site-specific mutagenesis of HIV-1(LAI). The K65R mutation also conferred greater than threefold cross-resistance to 2',3'-dideoxycytidine, 2', 3'-dideoxyinosine, 2',3'-dideoxy-3'-thiacytidine, 9-(2-phosphonylmethoxyethyl)adenine, 2-amino-6-chloropurine dioxolane, dioxolanyl-5-fluorocytosine, and diaminopurine dioxolane but had only marginal effects on 3'-azido-3'-deoxthymidine (AZT) susceptibility. However, when introduced into a genetic background for AZT resistance (D67N, K70R, T215Y, T219Q), the K65R mutation reversed the AZT resistance. DNA sequencing of RT clones derived from the second resistant isolate identified the L74V mutation, previously reported to cause ddI resistance. The L74V mutation also decreased the AZT resistance when the mutation was introduced into a genetic background for AZT resistance (D67N, K70R, T215Y, T219Q) but to a lesser degree than the K65R mutation did. These findings indicate that DXG and certain 2',3'-dideoxy compounds (e.g., ddI) can select for the same resistance mutations and thus may not be optimal for use in combination. However, the combination of AZT with DXG or its orally bioavailable prodrug (-)-beta-D-2, 6-diaminopurine-dioxolane should be explored because of the suppressive effects of the K65R and L74V mutations on AZT resistance.
Figures
References
-
- Arion D, Kaushik N, McCormick S, Borkow G, Parniak M A. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry. 1998;37:15908–15917. - PubMed
-
- Balzarini J, Karlsson A, Perez-Perez M J, Camarasa M J, Tarpley W G, De Clercq E. Treatment of human immunodeficiency virus type 1 (HIV-1)-infected cells with combinations of HIV-1-specific inhibitors results in a different resistance pattern than does treatment with single-drug therapy. J Virol. 1993;67:5353–5359. - PMC - PubMed
-
- Buckheit R W, Kinjerski T L, Fliakas-Boltz V, Russell J D, Stup T L, Pallansch L A, Brouwer W G, Dao D C, Harrison W A, Schultz R J, Bader J P, Yang S S. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type 1-specific compounds related to oxathiin carboxanilide. Antimicrob Agents Chemother. 1995;39:2718–2727. - PMC - PubMed
-
- Condra J H, Schleif W A, Blahy O M, Gabryelski L, Graham D, Quintero J, Rhodes A, Robbins H, Roth E, Shivaprakash M, Titus D, Yang T, Teppler H, Squires K, Deutsch P, Emini E. In vivo emergence of HIV-1 to variants resistant to multiple protease inhibitors. Nature. 1995;374:569–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous