Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin
- PMID: 10858336
- PMCID: PMC89967
- DOI: 10.1128/AAC.44.7.1818-1824.2000
Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin
Abstract
The penetration of two antibiotics, ampicillin and ciprofloxacin, through biofilms developed in an in vitro model system was investigated. The susceptibilities of biofilms and corresponding freely suspended bacteria to killing by the antibiotics were also measured. Biofilms of Klebsiella pneumoniae were developed on microporous membranes resting on agar nutrient medium. The susceptibilities of planktonic cultures and biofilms to 10 times the MIC were determined. Antibiotic penetration through biofilms was measured by assaying the concentration of antibiotic that diffused through the biofilm to an overlying filter disk. Parallel experiments were performed with a mutant K. pneumoniae strain in which beta-lactamase activity was eliminated. For wild-type K. pneumoniae grown in suspension culture, ampicillin and ciprofloxacin MICs were 500 and 0.18 microgram/ml, respectively. The log reductions in the number of CFU of planktonic wild-type bacteria after 4 h of treatment at 10 times the MIC were 4.43 +/- 0.33 and 4.14 +/- 0.33 for ampicillin and ciprofloxacin, respectively. Biofilms of the same strain were much less susceptible, yielding log reductions in the number of CFU of -0.06 +/- 0.06 and 1.02 +/- 0.04 for ampicillin and ciprofloxacin, respectively, for the same treatment. The number of CFU in the biofilms after 24 h of antibiotic exposure was not statistically different from the number after 4 h of treatment. Ampicillin did not penetrate wild-type K. pneumoniae biofilms, whereas ciprofloxacin and a nonreactive tracer (chloride ion) penetrated the biofilms quickly. The concentration of ciprofloxacin reached the MIC throughout the biofilm within 20 min. Ampicillin penetrated biofilms formed by a beta-lactamase-deficient mutant. However, the biofilms formed by this mutant were resistant to ampicillin treatment, exhibiting a 0.18 +/- 0.07 log reduction in the number of CFU after 4 h of exposure and a 1.64 +/- 0.33 log reduction in the number of CFU after 24 h of exposure. Poor penetration contributed to wild-type biofilm resistance to ampicillin but not to ciprofloxacin. The increased resistance of the wild-type strain to ciprofloxacin and the mutant strain to ampicillin and ciprofloxacin could not be accounted for by antibiotic inactivation or slow diffusion since these antibiotics fully penetrated the biofilms. These results suggest that some other resistance mechanism is involved for both agents.
Figures

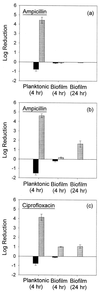
 ,
antibiotic-challenged cultures. Error bars reflect ±1 standard error
of the mean.
,
antibiotic-challenged cultures. Error bars reflect ±1 standard error
of the mean.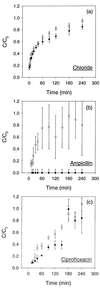

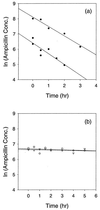
References
-
- American Public Health Association, American Water Works Association, and Water and Environment Federation. Standard methods for the examination of water and wastewater. Washington, D.C.: American Public Health Association; 1995.
-
- Bailey J E, Ollis D F. Biochemical engineering fundamentals. New York, N.Y: McGraw-Hill Book Co.; 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

