Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes
- PMID: 10859165
- PMCID: PMC316692
Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes
Abstract
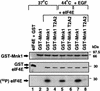
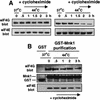
Inhibition of protein synthesis during heat shock limits accumulation of unfolded proteins that might damage eukaryotic cells. We demonstrate that chaperone Hsp27 is a heat shock-induced inhibitor of cellular protein synthesis. Translation of most mRNAs requires formation of a cap-binding initiation complex known as eIF4F, consisting of factors eIF4E, eIF4A, eIF4E kinase Mnk1, poly(A)-binding protein, and adaptor protein eIF4G. Hsp27 specifically bound eIF4G during heat shock, preventing assembly of the cap-initiation/eIF4F complex and trapping eIF4G in insoluble heat shock granules. eIF4G is a specific target of Hsp27, as eIF4E, eIF4A, Mnk1, poly(A)-binding protein, eIF4B, and eIF3 were not bound by Hsp27 and were not recruited into insoluble complexes. Dissociation of eIF4F was enhanced during heat shock by ectopic overexpression of Hsp25, the murine homolog of human Hsp27. Overexpression of Hsc70, a constitutive homolog of Hsp70, prevented loss of cap-initiation complexes and maintained eIF4G solubility. Purified Hsp27 specifically bound purified eIF4G in vitro, prevented in vitro translation, eliminated eIF4G interaction with protein binding factors, and promoted eIF4G insolubilization. These results therefore demonstrate that Hsp27 is a heat-induced inhibitor of eIF4F-dependent mRNA translation.
Figures






References
-
- Carper SW, Rocheleau T, Cimino D, Storm FK. Hsp 27 stimulates recovery of RNA and protein synthesis following a heat shock. J Cell Biochem. 1997;66:153–164. - PubMed
-
- Craig EA, Weissman JS, Horwich AL. Hsps and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. - PubMed
-
- Duncan RF. Translational control during heat shock. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 271–294.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
