Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2
- PMID: 10859199
- PMCID: PMC59037
- DOI: 10.1104/pp.123.2.689
Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2
Abstract
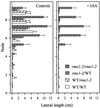
Decapitation-induced axillary bud outgrowth is a vital mechanism whereby shoots are able to continue normal growth and development. In many plants, including wild-type garden pea (Pisum sativum L.), this process can be inhibited by exogenous auxin. Using the ramosus (rms) increased branching mutants of pea, we present evidence that this response to auxin is dependent on graft-transmissible substance(s) regulated by the genes Rms1 and Rms2. The response to exogenous auxin is massively diminished in decapitated rms1 and rms2 mutant plants. However, basipetal auxin transport is not reduced in intact or decapitated mutants. Grafting rms1 or rms2 shoots onto wild-type rootstocks restored the auxin response, indicating that Rms1 and Rms2 gene action in the rootstock is sufficient to enable an auxin response in mutant shoots. We conclude that Rms1 and Rms2 act in the rootstock and shoot to control levels of mobile substance(s) that interact with exogenous auxin in the inhibition of bud outgrowth after decapitation. At least for rms1, the reduced auxin response is unlikely to be due to an inability of auxin to decrease xylem sap cytokinin content, as this is already low in intact rms1 plants. Consequently, we have genetic evidence that auxin action in decapitated plants depends on at least one novel long-distance signal.
Figures
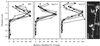

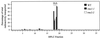


References
-
- Arumingtyas EL, Floyd RS, Gregory MJ, Murfet IC. Branching in Pisum: inheritance and allelism tests with 17 ramosus mutants. Pisum Genet. 1992;24:17–31.
-
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta. 1994;194:439–442.
-
- Beveridge CA (2000) Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul (in press)
-
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J. 1997a;11:339–345.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

