Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase
- PMID: 10859201
- PMCID: PMC59039
- DOI: 10.1104/pp.123.2.711
Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase
Abstract
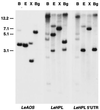
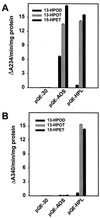
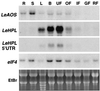
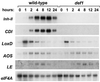
Allene oxide synthase (AOS) and fatty acid hydroperoxide lyase (HPL) are plant-specific cytochrome P450s that commit fatty acid hydroperoxides to different branches of oxylipin metabolism. Here we report the cloning and characterization of AOS (LeAOS) and HPL (LeHPL) cDNAs from tomato (Lycopersicon esculentum). Functional expression of the cDNAs in Escherichia coli showed that LeAOS and LeHPL encode enzymes that metabolize 13- but not 9-hydroperoxide derivatives of C(18) fatty acids. LeAOS was active against both 13S-hydroperoxy-9(Z),11(E),15(Z)-octadecatrienoic acid (13-HPOT) and 13S-hydroperoxy-9(Z),11(E)-octadecadienoic acid, whereas LeHPL showed a strong preference for 13-HPOT. These results suggest a role for LeAOS and LeHPL in the metabolism of 13-HPOT to jasmonic acid and hexenal/traumatin, respectively. LeAOS expression was detected in all organs of the plant. In contrast, LeHPL expression was predominant in leaves and flowers. Damage inflicted to leaves by chewing insect larvae led to an increase in the local and systemic expression of both genes, with LeAOS showing the strongest induction. Wound-induced expression of LeAOS also occurred in the def-1 mutant that is deficient in octadecanoid-based signaling of defensive proteinase inhibitor genes. These results demonstrate that tomato uses genetically distinct signaling pathways for the regulation of different classes of wound responsive genes.
Figures




References
-
- Baier M, Dietz K-J. Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci. 1999;4:166–168. - PubMed
-
- Bate NJ, Rothstein SJ. C6-Volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

