Inhibition of plant asparagine synthetase by monoterpene cineoles
- PMID: 10859202
- PMCID: PMC59040
- DOI: 10.1104/pp.123.2.725
Inhibition of plant asparagine synthetase by monoterpene cineoles
Retraction in
-
Inhibition of plant asparagine synthetase by monoterpene cineoles.Plant Physiol. 2005 Apr;137(4):1487. doi: 10.1104/pp.104.900143. Plant Physiol. 2005. PMID: 15824288 Free PMC article. No abstract available.
Abstract
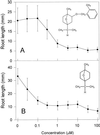
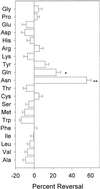
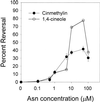
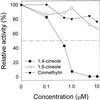
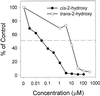
Asparagine (Asn) synthetase (AS) is the key enzyme in Asn biosynthesis and plays an important role in nitrogen mobilization. Despite its important physiological function, little research has been done documenting inhibitors of plant AS. Plant growth inhibition caused by the natural monoterpene 1,4-cineole and its structurally related herbicide cinmethylin was reversed 65% and 55%, respectively, by providing 100 microM Asn exogenously. Reversion of the phytotoxic effect was dependent on the concentration of Asn. The presence of either 1,4-cineole or cinmethylin stimulated root uptake of [(14)C]Asn by lettuce (Lactuca sativa) seedlings. Although the physiological responses suggested that both compounds affected Asn biosynthesis, biochemical analysis of AS activity showed that the natural monoterpene was a potent inhibitor (I(50) = approximately 0. 5 microM) of the enzyme, whereas the commercial product was not inhibitory up to levels of 10 mM. Analysis of the putative metabolite, 2-hydroxy-1,4-cineole, showed that the cis-enantiomer was much more active than the trans-enantiomer, suggesting that the hydroxyl group was involved in the specific ligand/active site interaction. This is the first report that AS is a suitable herbicide target site, and that cinmethylin is apparently a proherbicide that requires metabolic bioactivation via cleavage of the benzyl-ether side chain.
Figures


References
-
- Ahmad A, Misra LN. Terpenoids from Artemisia annua and constituents of its essential oil. Phytochemistry. 1994;37:183–186.
-
- Amagasa T, Paul RN, Heitholt JJ, Duke SO. Physiological effects of cornexistin on Lemna pausicostata. Pestic Biochem Physiol. 1994;49:37–42.
-
- Asplund RO. Monoterpenes: relationship between structure and inhibition of germination. Phytochemistry. 1968;7:1995–1997.
-
- Baum SF, Karanastasis L, Rost TL. Morphogenetic effect of the herbicide Cinch on Arabidopsis thaliana root development. J Plant Growth Regul. 1998;17:107–114.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

