Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors
- PMID: 10859349
- PMCID: PMC16683
- DOI: 10.1073/pnas.120035997
Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors
Abstract
Kinins are important mediators in cardiovascular homeostasis, inflammation, and nociception. Two kinin receptors have been described, B1 and B2. The B2 receptor is constitutively expressed, and its targeted disruption leads to salt-sensitive hypertension and altered nociception. The B1 receptor is a heptahelical receptor distinct from the B2 receptor in that it is highly inducible by inflammatory mediators such as bacterial lipopolysaccharide and interleukins. To clarify its physiological function, we have generated mice with a targeted deletion of the gene for the B1 receptor. B1 receptor-deficient animals are healthy, fertile, and normotensive. In these mice, bacterial lipopolysaccharide-induced hypotension is blunted, and there is a reduced accumulation of polymorphonuclear leukocytes in inflamed tissue. Moreover, under normal noninflamed conditions, they are analgesic in behavioral tests of chemical and thermal nociception. Using whole-cell patch-clamp recordings, we show that the B1 receptor was not necessary for regulating the noxious heat sensitivity of isolated nociceptors. However, by using an in vitro preparation, we could show that functional B1 receptors are present in the spinal cord, and their activation can facilitate a nociceptive reflex. Furthermore, in B1 receptor-deficient mice, we observed a reduction in the activity-dependent facilitation (wind-up) of a nociceptive spinal reflex. Thus, the kinin B1 receptor plays an essential physiological role in the initiation of inflammatory responses and the modulation of spinal cord plasticity that underlies the central component of pain. The B1 receptor therefore represents a useful pharmacological target especially for the treatment of inflammatory disorders and pain.
Figures
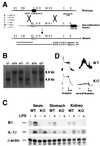
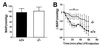
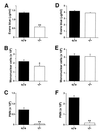

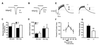
Comment in
-
Toward a new anti-inflammatory and analgesic agent.Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):7676-7. doi: 10.1073/pnas.150233397. Proc Natl Acad Sci U S A. 2000. PMID: 10869442 Free PMC article. No abstract available.
References
-
- Bhoola K D, Figueroa C D, Worthy K. Pharmacol Rev. 1992;44:1–80. - PubMed
-
- Borkowski J A, Ransom R W, Seabrook G R, Trumbauer M, Chen H, Hill R G, Strader C D, Hess J F. J Biol Chem. 1995;270:13706–13710. - PubMed
-
- Rupniak N M, Boyce S, Webb J K, Williams A R, Carlson E J, Hill R G, Borkowski J A, Hess J F. Pain. 1997;71:89–97. - PubMed
-
- Madeddu P, Varoni M V, Palomba D, Emanueli C, Demontis M P, Glorioso N, Dessi Fulgheri P, Sarzani R, Anania V. Circulation. 1997;96:3570–3578. - PubMed
-
- Ahluwalia A, Perretti M. J Immunol. 1996;156:269–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

