Identification and characterization of a newly recognized population of high-Na+, low-K+, low-density sickle and normal red cells
- PMID: 10859357
- PMCID: PMC16667
- DOI: 10.1073/pnas.130198797
Identification and characterization of a newly recognized population of high-Na+, low-K+, low-density sickle and normal red cells
Abstract
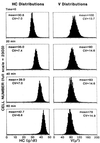
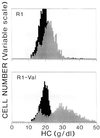
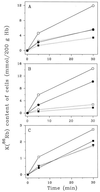
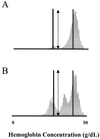
We describe a population of sickle cell anemia red cells (SS RBCs) ( approximately 4%) and a smaller fraction of normal RBCs (<0.03%) that fail to dehydrate when permeabilized to K(+) with either valinomycin or elevated internal Ca(2+). The nonshrinking, valinomycin-resistant (val-res) fractions, first detected by flow cytometry of density-fractionated SS RBCs, constituted up to 60% of the lightest, reticulocyte-rich (R1) cell fraction, and progressively smaller portions of the slightly denser R2 cells and discocytes. R1 val-res RBCs had a mean cell hemoglobin concentration of approximately 21 g of Hb per dl, and many had an elongated shape like "irreversibly sickled cells," suggesting a dense SS cell origin. Of three possible explanations for val-res cells, failure of valinomycin to K(+)-permeabilize the cells, low co-ion permeability, or reduced driving K(+) gradient, the latter proved responsible: Both SS and normal val-res RBCs were consistently high-Na(+) and low-K(+), even when processed entirely in Na-free media. Ca(2+) + A23187-induced K(+)-permeabilization of SS R1 fractions revealed a similar fraction of cal-res cells, whose (86)Rb uptake showed both high Na/K pump and leak fluxes. val-res/cal-res RBCs might represent either a distinct erythroid genealogy, or an "end-stage" of normal and SS RBCs. This paper focuses on the discovery, basic characterization, and exclusion of artifactual origin of this RBC fraction. Many future studies will be needed to clarify their mechanism of generation and full pathophysiological significance.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

