Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy
- PMID: 10859361
- PMCID: PMC16666
- DOI: 10.1073/pnas.140123497
Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy
Abstract
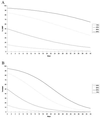
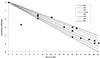
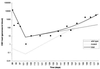
Cytomegalovirus (CMV) replicates rapidly in its human host with a doubling time of approximately 1 day. Using simple mathematical models we show that the efficacy of the anti-CMV drug ganciclovir (GCV) against wild-type strains is 91.5% [95% confidence interval (CI) 89-94%] when given i.v. (5 mg/kg twice a day) but only 46.5% (95% CI 45-47.5%) when given orally (1 g three times a day) whereas the corresponding figures for a typical GCV-resistant virus are 62% (95% CI 57-66%) and 35% (95% CI 33-37%), respectively. During prolonged periods of GCV therapy we show that the apparent sudden appearance of GCV resistance is explained by the combination of two exponentially increasing populations (wild type and mutant) at doses of GCV that do not completely inhibit CMV replication. Cell culture methods to assess CMV drug resistance in vivo will underestimate its prevalence because of the fitness loss of resistant virus in the absence of therapy. The parameters determined from these models then were used to predict the likely viral load and resistance patterns in patients on prolonged therapy with GCV. The modeled and experimental data showed excellent agreement over extended time periods (up to 270 days of therapy) and provide a framework to predict the virologic course of patients at therapeutic initiation.
Figures




References
-
- Griffiths P D, Emery V C. In: Clinical Virology. Richman D D, Whitley R J, Hayden F G, editors. New York: Churchill Livingstone; 1997. pp. 445–470.
-
- Fishman J A, Rubin R H. N Engl J Med. 1998;338:1741–1751. - PubMed
-
- Crumpacker C S. N Engl J Med. 1996;335:721–729. - PubMed
-
- Safrin S, Cherrington J, Jaffe H S. Rev Med Virol. 1997;7:145–156. - PubMed
-
- Jacobsen M A. N Engl J Med. 1997;337:105–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

