Toward antibody-catalyzed hydrolysis of organophosphorus poisons
- PMID: 10860971
- PMCID: PMC16498
- DOI: 10.1073/pnas.97.13.7058
Toward antibody-catalyzed hydrolysis of organophosphorus poisons
Abstract
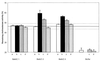
We report here our preliminary results on the use of catalytic antibodies as an approach to neutralizing organophosphorus chemical weapons. A first-generation hapten, methyl-alpha-hydroxyphosphinate Ha, was designed to mimic the approach of an incoming water molecule for the hydrolysis of exceedingly toxic methylphosphonothioate VX (1a). A moderate protective activity was first observed on polyclonal antibodies raised against Ha. The results were further confirmed by using a mAb PAR 15 raised against phenyl-alpha-hydroxyphosphinate Hb, which catalyzes the hydrolysis of PhX (1b), a less toxic phenylphosphonothioate analog of VX with a rate constant of 0.36 M(-1) x min(-1) at pH 7.4 and 25 degrees C, which corresponds to a catalytic proficiency of 14,400 M(-1) toward the rate constant for the uncatalyzed hydrolysis of 1b. This is a demonstration on the organophosphorus poisons themselves that mAbs can catalytically hydrolyze nerve agents, and a significant step toward the production of therapeutically active abzymes to treat poisoning by warfare agents.
Figures
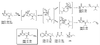

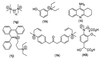

References
-
- Taylor P. In: Anticholinesterase Agent in the Pharmacological Basis of Therapeutics. Goodman Gilman A, Rall T W, Nied A S, Taylor P, editors. New York: Pergamon; 1990.
-
- Bunton C A. In: Chemical Warfare in Macmillan Encyclopedia of Chemistry. Lagowski J J, editor. Vol. 1. New York: Macmillan; 1997. pp. 343–346.
-
- Yang Y C, Baker J A, Ward J R. Chem Rev. 1992;92:1729–1743.
-
- Millard C B, Lokridge O, Broomfield C A. Biochemistry. 1995;34:15925–15933. - PubMed
-
- Millard C B, Lokridge O, Broomfield C A. Biochemistry. 1998;37:237–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

