Identification and characterization of a p53 homologue in Drosophila melanogaster
- PMID: 10860994
- PMCID: PMC16540
- DOI: 10.1073/pnas.97.13.7301
Identification and characterization of a p53 homologue in Drosophila melanogaster
Abstract
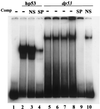
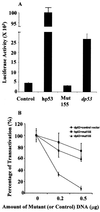
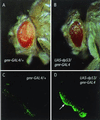
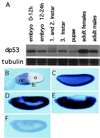
The tumor suppressor gene p53 in mammalian cells plays a critical role in safeguarding the integrity of genome. It functions as a sequence-specific transcription factor. Upon activation by a variety of cellular stresses, p53 transactivates downstream target genes, through which it regulates cell cycle and apoptosis. However, little is known about p53 in invertebrates. Here we report the identification and characterization of a Drosophila p53 homologue gene, dp53. dp53 encodes a 385-amino acid protein with significant homology to human p53 (hp53) in the region of the DNA-binding domain, and to a lesser extent the tetramerization domain. Purified dp53 DNA-binding domain protein was shown to bind to the consensus hp53-binding site by gel mobility analysis. In transient transfection assays, expression of dp53 in Schneider cells transcriptionally activated promoters that contained consensus hp53-responsive elements. Moreover, a mutant dp53 (Arg-155 to His-155), like its hp53 counterpart mutant, exerted a dominant-negative effect on transactivation. Ectopic expression of dp53 in Drosophila eye disk caused cell death and led to a rough eye phenotype. dp53 is expressed throughout the development of Drosophila with highest expression levels in early embryogenesis, which has a maternal component. Consistent with this, dp53 RNA levels were high in the nurse cells of the ovary. It appears that p53 is structurally and functionally conserved from flies to mammals. Drosophila will provide a useful genetic system to the further study of the p53 network.
Figures

References
-
- Levine A J. Cell. 1997;88:323–331. - PubMed
-
- Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. - PubMed
-
- Oliner J D, Kinzler K W, Meltzer P S, George D L, Vogelstein B. Nature (London) 1992;358:80–83. - PubMed
-
- Kussie P H, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine A J, Pavletich N P. Science. 1996;274:948–953. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

