Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes
- PMID: 10861084
- PMCID: PMC4967413
- DOI: 10.4049/jimmunol.165.1.453
Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes
Abstract
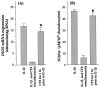
Inflammatory cytokines play a major role in cartilage destruction in diseases such as osteoarthritis and rheumatoid arthritis. Because physical therapies such as continuous passive motion yield beneficial effects on inflamed joints, we examined the intracellular mechanisms of mechanical strain-mediated actions in chondrocytes. By simulating the effects of continuous passive motion with cyclic tensile strain (CTS) on chondrocytes in vitro, we show that CTS is a potent antagonist of IL-1 beta actions and acts as both an anti-inflammatory and a reparative signal. Low magnitude CTS suppresses IL-1 beta-induced mRNA expression of multiple proteins involved in catabolic responses, such as inducible NO synthase, cyclo-oxygenase II, and collagenase. CTS also counteracts cartilage degradation by augmenting mRNA expression for tissue inhibitor of metalloproteases and collagen type II that are inhibited by IL-1 beta. Additionally, CTS augments the reparative process via hyperinduction of aggrecan mRNA expression and abrogation of IL-1 beta-induced suppression of proteoglycan synthesis. Nonetheless, the presence of an inflammatory signal is a prerequisite for the observed CTS actions, as exposure of chondrocytes to CTS alone has little effect on these parameters. Functional analysis suggests that CTS-mediated anti-inflammatory actions are not mediated by IL-1R down-regulation. Moreover, as an effective antagonist of IL-1 beta, the actions of CTS may involve disruption/regulation of signal transduction cascade of IL-1 beta upstream of mRNA transcription. These observations are the first to show that CTS directly acts as an anti-inflammatory signal on chondrocytes and provide a molecular basis for its actions.
Figures
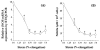

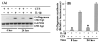



References
-
- Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin. Arthritis Rheum. 1998;27:392. - PubMed
-
- Bandara G, Georgescu HI, Lin CW, Evans CH. Synovial activation of chondrocytes: evidence for complex cytokine interactions. Agents Actions. 1991;34:285. - PubMed
-
- Flugge LA, Miller-Deist LA, Petillo PA. Towards a molecular understanding of arthritis. Chem. Biol. 1999;6:R157. - PubMed
-
- van Den Berg WB. Lessons for joint destruction from animal models. Curr. Opin. Rheumatol. 1997;9:221. - PubMed
-
- Firstein GS, Boyle DL, Yu C, Paine MM, Whisenand TD, Svaifler NJ, Aredn WP. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994;37:644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

