Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity
- PMID: 10862788
- PMCID: PMC378505
- DOI: 10.1172/JCI8472
Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity
Abstract
The immune response to oxidized LDL (OxLDL) may play an important role in atherogenesis. Working with apoE-deficient mice, we isolated a panel of OxLDL-specific B-cell lines that secrete IgM Abs that specifically bind to oxidized phospholipids such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC). These Abs block uptake of OxLDL by macrophages, recognize similar oxidation-specific epitopes on apoptotic cells, and are deposited in atherosclerotic lesions. The Abs were found to be structurally and functionally identical to classic "natural" T15 anti-PC Abs that are of B-1 cell origin and are reported to provide optimal protection from virulent pneumococcal infection. These findings suggest that there has been natural selection for B-1 cells secreting oxidation-specific/T15 antibodies, both for their role in natural immune defense and for housekeeping roles against oxidation-dependent neodeterminants in health and disease.
Figures
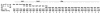
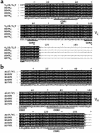





Comment in
-
Immune recognition of OxLDL in atherosclerosis.J Clin Invest. 2000 Jun;105(12):1683-5. doi: 10.1172/JCI10426. J Clin Invest. 2000. PMID: 10862783 Free PMC article. Review. No abstract available.
References
-
- Steinberg, D., and Witztum, J.L. 1999. Lipoproteins, lipoprotein oxidation, and atherogenesis. In Molecular basis of cardiovascular disease. K.R. Chien, editor. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 458–475.
-
- Palinski W, et al. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. - PubMed
-
- Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

