Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells
- PMID: 10862792
- PMCID: PMC378507
- DOI: 10.1172/JCI8892
Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells
Abstract
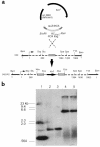
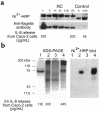
Enteroaggregative Escherichia coli (EAEC) is an emerging cause of acute and persistent diarrhea worldwide. EAEC infections are associated with intestinal inflammation and growth impairment in infected children, even in the absence of diarrhea. We previously reported that prototype EAEC strains rapidly induce IL-8 production by Caco-2 intestinal epithelial cells, and that this effect is mediated by a soluble, heat-stable factor released by these bacteria in culture. We herein report the cloning, sequencing, and expression of this biologically active IL-8-releasing factor from EAEC, and its identification as a flagellin that is unique among known expressed proteins. Flagella purified from EAEC 042 and several other EAEC isolates potently release IL-8 from Caco-2 cells; an engineered aflagellar mutant of 042 does not release IL-8. Finally, cloned EAEC flagellin expressed in nonpathogenic E. coli as a polyhistidine-tagged fusion protein maintains its proinflammatory activity. These findings demonstrate a major new means by which EAEC may cause intestinal inflammation, persistent diarrhea, and growth impairment that characterize human infection with these organisms. Furthermore, they open new approaches for diagnosis and vaccine development. This novel pathogenic mechanism of EAEC extends an emerging paradigm of bacterial flagella as inflammatory stimuli.
Figures
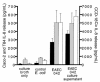


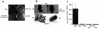

Comment in
-
Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens.J Clin Invest. 2001 Jan;107(1):27-30. doi: 10.1172/JCI11792. J Clin Invest. 2001. PMID: 11134175 Free PMC article. Review. No abstract available.
References
-
- Nataro J, et al. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. - PubMed
-
- Gascon J, et al. Enteroaggregative Escherichia coli strains as a cause of traveler’s diarrhea: a case-control study. J Infect Dis. 1998;177:1409–1412. - PubMed
-
- Mayer HB, Wanke CA. Enteroaggregative Escherichia coli as a possible cause of diarrhea in an HIV-infected patient. N Engl J Med. 1995;332:273–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

