Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics
- PMID: 10864641
- PMCID: PMC112137
- DOI: 10.1128/jvi.74.14.6316-6323.2000
Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics
Abstract
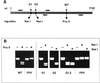
The hemagglutinin (HA) of fowl plague virus A/FPV/Rostock/34 (H7N1) carries two N-linked oligosaccharides attached to Asn123 and Asn149 in close vicinity to the receptor-binding pocket. In previous studies in which HA mutants lacking either one (mutants G1 and G2) or both (mutant G1,2) glycosylation sites had been expressed from a simian virus 40 vector, we showed that these glycans regulate receptor binding affinity (M. Ohuchi, R. Ohuchi, A. Feldmann, and H. D. Klenk, J. Virol. 71:8377-8384, 1997). We have now investigated the effect of these mutations on virus growth using recombinant viruses generated by an RNA polymerase I-based reverse genetics system. Two reassortants of influenza virus strain A/WSN/33 were used as helper viruses to obtain two series of HA mutant viruses differing only in the neuraminidase (NA). Studies using N1 NA viruses revealed that loss of the oligosaccharide from Asn149 (mutant G2) or loss of both oligosaccharides (mutant G1,2) has a pronounced effect on virus growth in MDCK cells. Growth of virus lacking both oligosaccharides from infected cells was retarded, and virus yields in the medium were decreased about 20-fold. Likewise, there was a reduction in plaque size that was distinct with G1,2 and less pronounced with G2. These effects could be attributed to a highly impaired release of mutant progeny viruses from host cells. In contrast, with recombinant viruses containing N2 NA, these restrictions were much less apparent. N1 recombinants showed lower neuraminidase activity than N2 recombinants, indicating that N2 NA is able to partly overrule the high-affinity binding of mutant HA to the receptor. These results demonstrate that N-glycans flanking the receptor-binding site of the HA molecule are potent regulators of influenza virus growth, with the glycan at Asn149 being dominant and that at Asn123 being less effective. In addition, we show here that HA and NA activities need to be highly balanced in order to allow productive influenza virus infection.
Figures






References
-
- Colman P. Structure and function of the neuraminidase. In: Nicholson K G, Webster R G, Hay A J, editors. Textbook of influenza. London, England: Blackwell Science; 1998. pp. 65–73.
-
- Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

