Human papillomavirus type 16 E6 induces self-ubiquitination of the E6AP ubiquitin-protein ligase
- PMID: 10864652
- PMCID: PMC112148
- DOI: 10.1128/jvi.74.14.6408-6417.2000
Human papillomavirus type 16 E6 induces self-ubiquitination of the E6AP ubiquitin-protein ligase
Abstract
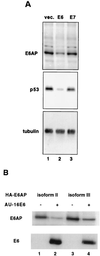
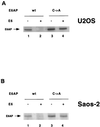
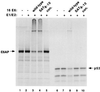
The E6 protein of the high-risk human papillomaviruses (HPVs) and the cellular ubiquitin-protein ligase E6AP form a complex which causes the ubiquitination and degradation of p53. We show here that HPV16 E6 promotes the ubiquitination and degradation of E6AP itself. The half-life of E6AP is shorter in HPV-positive cervical cancer cells than in HPV-negative cervical cancer cells, and E6AP is stabilized in HPV-positive cancer cells when expression of the viral oncoproteins is repressed. Expression of HPV16 E6 in cells results in a threefold decrease in the half-life of transfected E6AP. E6-mediated degradation of E6AP requires (i) the binding of E6 to E6AP, (ii) the catalytic activity of E6AP, and (iii) activity of the 26S proteasome, suggesting that E6-E6AP interaction results in E6AP self-ubiquitination and degradation. In addition, both in vitro and in vivo experiments indicate that E6AP self-ubiquitination results primarily from an intramolecular transfer of ubiquitin from the active-site cysteine to one or more lysine residues; however, intermolecular transfer can also occur in the context of an E6-mediated E6AP multimer. Finally, we demonstrate that an E6 mutant that is able to immortalize human mammary epithelial cells but is unable to degrade p53 retains its ability to bind and degrade E6AP, raising the possibility that E6-mediated degradation of E6AP contributes to its ability to transform mammalian cells.
Figures





References
-
- Andersson S, Davis D L, Dahlback H, Jornvall H, Russell D W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
-
- Beer-Romero P, Glass S, Rolfe M. Antisense targeting of E6AP elevates p53 in HPV-infected cells but not in normal cells. Oncogene. 1997;14:595–602. - PubMed
-
- Boyer S N, Wazer D E, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

