Infection of polarized cultures of human intestinal epithelial cells with hepatitis A virus: vectorial release of progeny virions through apical cellular membranes
- PMID: 10864660
- PMCID: PMC112156
- DOI: 10.1128/jvi.74.14.6476-6484.2000
Infection of polarized cultures of human intestinal epithelial cells with hepatitis A virus: vectorial release of progeny virions through apical cellular membranes
Abstract
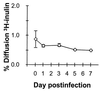
Although hepatitis A virus (HAV) is typically transmitted by the fecal-oral route, little is known of its interactions with cells of the gastrointestinal tract. We studied the replication of HAV in polarized cultures of Caco-2 cells, a human cell line which retains many differentiated functions of small intestinal epithelial cells. Virus uptake was 30- to 40-fold more efficient when the inoculum was placed on the apical rather than the basolateral surface of these cells, suggesting a greater abundance of the cellular receptor for HAV on the apical surface. Infection proceeded without cytopathic effect and did not influence transepithelial resistance or the diffusion of inulin across cell monolayers. Nonetheless, there was extensive release of progeny virus, which occurred almost exclusively into apical supernatant fluids (36.4% +/- 12.5% of the total virus yield compared with 0.23% +/- 0.13% release into basolateral fluids). Brefeldin A caused a profound inhibition of HAV replication, but also selectively reduced apical release of virus. These results indicate that polarized human epithelial cell cultures undergo vectorial infection with HAV and that virus release is largely restricted to the apical membrane. Virus release occurs in the absence of cytopathic effect and may involve cellular vesicular transport mechanisms.
Figures



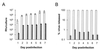



References
-
- Asher L V S, Binn L N, Mensing T L, Marchwicki R H, Vassell R A, Young G D. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivergatus) J Med Virol. 1995;47:260–268. - PubMed
-
- Blau D M, Compans R W. Polarization of viral entry and release in epithelial cells. Semin Virol. 1996;7:245–253.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

