Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons
- PMID: 10864940
- PMCID: PMC6772270
- DOI: 10.1523/JNEUROSCI.20-13-04829.2000
Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons
Abstract
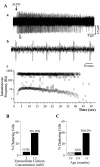
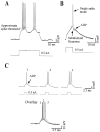
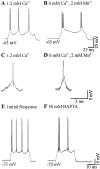
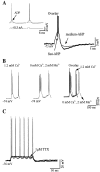
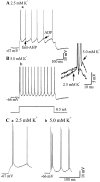
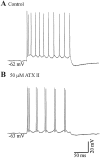
Neocortical neurons in awake, behaving animals can generate high-frequency (>300 Hz) bursts of action potentials, either in single bursts or in a repetitive manner. Intracellular recordings of layer II/III pyramidal neurons were obtained from adult ferret visual cortical slices maintained in vitro to investigate the ionic mechanisms by which a subgroup of these cells generates repetitive, high-frequency burst discharges, a pattern referred to as "chattering." The generation of each but the first action potential in a burst was dependent on the critical interplay between the afterhyperpolarizations (AHPs) and afterdepolarizations (ADPs) that followed each action potential. The spike-afterdepolarization and the generation of action potential bursts were dependent on Na(+), but not Ca(2+), currents. Neither blocking of the transmembrane flow of Ca(2+) nor the intracellular chelation of free Ca(2+) with BAPTA inhibited the generation of intrinsic bursts. In contrast, decreasing the extracellular Na(+) concentration or pharmacologically blocking Na(+) currents with tetrodotoxin, QX-314, or phenytoin inhibited bursting before inhibiting action potential generation. Additionally, a subset of layer II/III pyramidal neurons could be induced to switch from repetitive single spiking to a burst-firing mode by constant depolarizing current injection, by raising extracellular K(+) concentrations, or by potentiation of the persistent Na(+) current with the Na(+) channel toxin ATX II. These results indicate that cortical neurons may dynamically regulate their pattern of action potential generation through control of Na(+) and K(+) currents. The generation of high-frequency burst discharges may strongly influence the response of postsynaptic neurons and the operation of local cortical networks.
Figures








References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Agmon A, Connors BW. Repetitive burst-firing in the deep layers of mouse somatosensory cortex. Neurosci Lett. 1989;99:137–141. - PubMed
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Whitteridge D. Estimates of the net excitatory currents evoked by visual stimulation of identified neurons in cat visual cortex. Cereb Cortex. 1998;8:462–476. - PubMed
-
- Alsen C. Biological significance of peptides from Anemonia sulcata. FASEB J. 1983;42:101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
