Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training
- PMID: 10864975
- PMCID: PMC6772299
- DOI: 10.1523/JNEUROSCI.20-13-05179.2000
Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training
Abstract
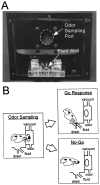
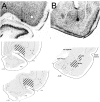
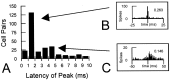
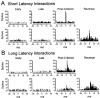
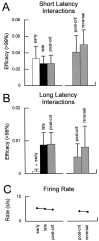
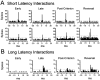
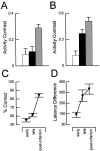
Interconnections between orbitofrontal cortex (OFC) and basolateral amygdala (ABL) are critical for encoding and using associative information about the motivational significance of stimuli. Previously, we reported that neurons in OFC and ABL fired selectively to cues during odor discrimination learning and reversal training. Here we conducted an analysis of correlated firing in the cell pairs recorded in the previous study. Correlated firing during the intertrial intervals was compared across task phases during different phases of acquisition and reversal learning. Changes in correlated activity during initial learning and subsequent accurate performance on the discrimination problems closely resembled the changes in odor selectivity in OFC and ABL reported earlier. Increased correlated firing was most pronounced in OFC during accurate go, no-go performance in the postcriterion phase of performance, whereas correlated firing in ABL increased primarily during an earlier phase of learning. In contrast, findings during subsequent reversal training diverged from our earlier report in which odor selectivity diminished in OFC and reversed in ABL. When the reinforcement contingencies of the odors were reversed after the rat had learned the original associations, correlated firing further increased significantly in OFC but remained stable in ABL. This evidence that associative encoding increments with reversal learning in OFC suggests that the original associations, although not expressed as stimulus driven activity, may be maintained within the network as new associations are acquired.
Figures

References
-
- Abeles M. Quantification, smoothing, and confidence limits for single-units' histograms. J Neurosci Methods. 1982;5:317–325. - PubMed
-
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1294. - PubMed
-
- Brody CD. Correlations without synchrony. Neural Comput. 1999;11:1573–1551. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
