Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa
- PMID: 10869085
- PMCID: PMC94592
- DOI: 10.1128/JB.182.14.4051-4058.2000
Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa
Abstract
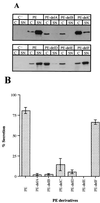
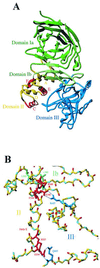
Pseudomonas aeruginosa is a gram-negative bacterium that secretes many proteins into the extracellular medium via the Xcp machinery. This pathway, conserved in gram-negative bacteria, is called the type II pathway. The exoproteins contain information in their amino acid sequence to allow targeting to their secretion machinery. This information may be present within a conformational motif. The nature of this signal has been examined for P. aeruginosa exotoxin A (PE). Previous studies failed to identify a common minimal motif required for Xcp-dependent recognition and secretion of PE. One study identified a motif at the N terminus of the protein, whereas another one found additional information at the C terminus. In this study, we assess the role of the central PE domain II composed of six alpha-helices (A to F). The secretion behavior of PE derivatives, individually deleted for each helix, was analyzed. Helix E deletion has a drastic effect on secretion of PE, which accumulates within the periplasm. The conformational rearrangement induced in this variant is predicted from the three-dimensional PE structure, and the molecular modification is confirmed by gel filtration experiments. Helix E is in the core of the molecule and creates close contact with other domains (I and III). Deletion of the surface-exposed helix F has no effect on secretion, indicating that no secretion information is contained in this helix. Finally, we concluded that disruption of a structured domain II yields an extended form of the molecule and prevents formation of the conformational secretion motif.
Figures




Similar articles
-
Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli.Proc Natl Acad Sci U S A. 1988 May;85(9):2939-43. doi: 10.1073/pnas.85.9.2939. Proc Natl Acad Sci U S A. 1988. PMID: 3283735 Free PMC article.
-
A deletion within the translocation domain of Pseudomonas exotoxin A enhances translocation efficiency and cytotoxicity concomitantly.Mol Microbiol. 1999 Mar;31(5):1385-93. doi: 10.1046/j.1365-2958.1999.01280.x. Mol Microbiol. 1999. PMID: 10200959
-
A specific targeting domain in mature exotoxin A is required for its extracellular secretion from Pseudomonas aeruginosa.EMBO J. 1996 Jan 15;15(2):429-36. EMBO J. 1996. PMID: 8617218 Free PMC article.
-
Redirecting Pseudomonas exotoxin.Semin Cell Biol. 1991 Feb;2(1):31-7. Semin Cell Biol. 1991. PMID: 1954341 Review.
-
Analysis of the structure-function relationship of Pseudomonas aeruginosa exotoxin A.Mol Microbiol. 1990 Apr;4(4):527-35. doi: 10.1111/j.1365-2958.1990.tb00620.x. Mol Microbiol. 1990. PMID: 2112672 Review.
Cited by
-
Exchange of Xcp (Gsp) secretion machineries between Pseudomonas aeruginosa and Pseudomonas alcaligenes: species specificity unrelated to substrate recognition.J Bacteriol. 2001 Feb;183(3):959-67. doi: 10.1128/JB.183.3.959-967.2001. J Bacteriol. 2001. PMID: 11208795 Free PMC article.
-
Protein Secretion Systems in Pseudomonas aeruginosa: An Essay on Diversity, Evolution, and Function.Front Microbiol. 2011 Jul 18;2:155. doi: 10.3389/fmicb.2011.00155. eCollection 2011. Front Microbiol. 2011. PMID: 21811488 Free PMC article.
-
Downsizing a pullulanase to a small molecule with improved soluble expression and secretion efficiency in Escherichia coli.Microb Cell Fact. 2016 Jan 14;15:9. doi: 10.1186/s12934-015-0403-5. Microb Cell Fact. 2016. PMID: 26762529 Free PMC article.
-
Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins.J Biol Chem. 2011 Apr 8;286(14):12317-27. doi: 10.1074/jbc.M110.193045. Epub 2011 Feb 16. J Biol Chem. 2011. PMID: 21325275 Free PMC article.
-
Bacterial secretins: Mechanisms of assembly and membrane targeting.Protein Sci. 2020 Apr;29(4):893-904. doi: 10.1002/pro.3835. Epub 2020 Feb 19. Protein Sci. 2020. PMID: 32020694 Free PMC article. Review.
References
-
- Bortoli-German I, Brun E, Py B, Chippaux M, Barras F. Periplasmic disulphide bond formation is essential for cellulase secretion by the plant pathogen Erwinia chrysanthemi. Mol Microbiol. 1994;11:545–553. - PubMed
-
- Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. - PubMed
-
- de Groot A, Gerritse G, Tommassen J, Lazdunski A, Filloux A. Molecular organization of the xcp gene cluster in Pseudomonas putida: absence of an xcpX (gspK) homologue. Gene. 1999;226:35–40. - PubMed
-
- Dums F, Dow J M, Daniels M J. Structural characterization of protein secretion genes of the bacterial phytopathogen Xanthomonas campestris pathovar campestris: relatedness to secretion systems of other gram-negative bacteria. Mol Gen Genet. 1991;229:357–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

