Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression
- PMID: 10869427
- PMCID: PMC16630
- DOI: 10.1073/pnas.130200197
Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression
Abstract
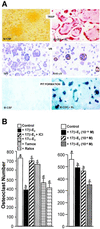
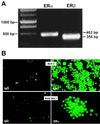
Loss of ovarian function following menopause results in a substantial increase in bone turnover and a critical imbalance between bone formation and resorption. This imbalance leads to a progressive loss of trabecular bone mass and eventually osteoporosis, in part the result of increased osteoclastogenesis. Enhanced formation of functional osteoclasts appears to be the result of increased elaboration by support cells of osteoclastogenic cytokines such as IL-1, tumor necrosis factor, and IL-6, all of which are negatively regulated by estrogens. We show here that estrogen can suppress receptor activator of NF-kappaB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)-induced differentiation of myelomonocytic precursors into multinucleated tartrate-resistant acid phosphatase-positive osteoclasts through an estrogen receptor-dependent mechanism that does not require mediation by stromal cells. This suppression is dose-dependent, isomer-specific, and reversed by ICI 182780. Furthermore, the bone-sparing analogues tamoxifen and raloxifene mimic estrogen's effects. Estrogen blocks RANKL/M-CSF-induced activator protein-1-dependent transcription, likely through direct regulation of c-Jun activity. This effect is the result of a classical nuclear activity by estrogen receptor to regulate both c-Jun expression and its phosphorylation by c-Jun N-terminal kinase. Our results suggest that estrogen modulates osteoclast formation both by down-regulating the expression of osteoclastogenic cytokines from supportive cells and by directly suppressing RANKL-induced osteoclast differentiation.
Figures



References
-
- Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. - PubMed
-
- Pacifici R. J Bone Miner Res. 1996;8:1043–1051. - PubMed
-
- Turner R T, Riggs B L, Spelsberg T C. Endocr Rev. 1994;15:275–300. - PubMed
-
- Roodman G D. Exp Hematol. 1999;27:1229–1241. - PubMed
-
- Jilka R L, Hangoc G, Girosole G, Passeri G, Williams D C, Abrams J S, Boyce B, Broxmeyer H, Manolagas S C. Science. 1992;257:88–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

