Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization
- PMID: 10869435
- PMCID: PMC16631
- DOI: 10.1073/pnas.140199597
Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization
Abstract
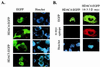
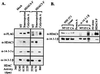
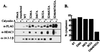
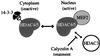
Transcription is controlled in part by the dynamic acetylation and deacetylation of histone proteins. The latter process is mediated by histone deacetylases (HDACs). Previous analysis of the regulation of HDAC activity in transcription has focused primarily on the recruitment of HDAC proteins to specific promoters or chromosomal domains by association with DNA-binding proteins. To characterize the cellular function of the recently identified HDAC4 and HDAC5 proteins, complexes were isolated by immunoprecipitation. Both HDACs were found to interact with14-3-3 proteins at three phosphorylation sites. The association of 14-3-3 with HDAC4 and HDAC5 results in the sequestration of these proteins in the cytoplasm. Loss of this interaction allows HDAC4 and HDAC5 to translocate to the nucleus, interact with HDAC3, and repress gene expression. Regulation of the cellular localization of HDAC4 and HDAC5 by 14-3-3 represents a mechanism for controlling the transcriptional activity of these class II HDAC proteins.
Figures

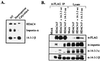


References
-
- Ayer D E. Trends Cell Biol. 1999;9:193–198. - PubMed
-
- Dangond F, Hafler D A, Tong J K, Randall J, Kojima R, Utku N, Gullans S R. Biochem Biophys Res Commun. 1998;242:648–652. - PubMed
-
- Fischle W, Emiliani S, Hendzel M J, Nagase T, Nomura N, Voelter W, Verdin E. J Biol Chem. 1999;274:11713–11720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

