Mechanism of action of a pestivirus antiviral compound
- PMID: 10869440
- PMCID: PMC16656
- DOI: 10.1073/pnas.140220397
Mechanism of action of a pestivirus antiviral compound
Abstract
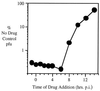
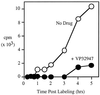
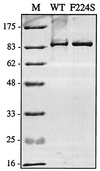
We report here the discovery of a small molecule inhibitor of pestivirus replication. The compound, designated VP32947, inhibits the replication of bovine viral diarrhea virus (BVDV) in cell culture at a 50% inhibitory concentration of approximately 20 nM. VP32947 inhibits both cytopathic and noncytopathic pestiviruses, including isolates of BVDV-1, BVDV-2, border disease virus, and classical swine fever virus. However, the compound shows no activity against viruses from unrelated virus groups. Time of drug addition studies indicated that VP32947 acts after virus adsorption and penetration and before virus assembly and release. Analysis of viral macromolecular synthesis showed VP32947 had no effect on viral protein synthesis or polyprotein processing but did inhibit viral RNA synthesis. To identify the molecular target of VP32947, we isolated drug-resistant (DR) variants of BVDV-1 in cell culture. Sequence analysis of the complete genomic RNA of two DR variants revealed a single common amino acid change located within the coding region of the NS5B protein, the viral RNA-dependent RNA polymerase. When this single amino acid change was introduced into an infectious clone of drug-sensitive wild-type (WT) BVDV-1, replication of the resulting virus was resistant to VP32947. The RNA-dependent RNA polymerase activity of the NS5B proteins derived from WT and DR viruses expressed and purified from recombinant baculovirus-infected insect cells confirmed the drug sensitivity of the WT enzyme and the drug resistance of the DR enzyme. This work formally validates NS5B as a target for antiviral drug discovery and development. The utility of VP32947 and similar compounds for the control of pestivirus diseases, and for hepatitis C virus drug discovery efforts, is discussed.
Figures
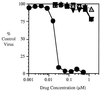
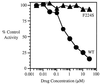
 ) viruses
in the presence of increasing concentrations of VP32947 was assessed in
a virus cytopathic effect assay. Data are plotted as the percent of
control virus cytopathic effect.
) viruses
in the presence of increasing concentrations of VP32947 was assessed in
a virus cytopathic effect assay. Data are plotted as the percent of
control virus cytopathic effect.
References
-
- Sockett D, Bolin D, Ridpath J, Bolin S. Bovine Viral Diarrhea Virus: A 50 Year Review. Ithaca, NY: Cornell University College of Veterinary Medicine; 1996. p. 207.
-
- Bitsch V, Ronsholt L. Vet Clin North Am Food Anim Pract. 1995;11:627–640. - PubMed
-
- Carman S, van Dreumel T, Ridpath J, Hazlett M, Alves D, Dubovi E, Tremblay R, Bolin S, Godkin A, Anderson N. J Vet Diagn Invest. 1998;10:27–35. - PubMed
-
- Herklots H, Nasto B. Am Soc Microbiol News. 1998;64:10.
-
- Houe H. Vet Microbiol. 1999;64:89–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

