The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana
- PMID: 10871276
- PMCID: PMC2175142
- DOI: 10.1083/jcb.149.7.1335
The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana
Abstract
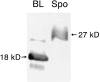
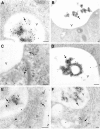
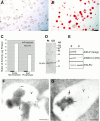
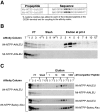
Many soluble plant vacuolar proteins are sorted away from secreted proteins into small vesicles at the trans-Golgi network by transmembrane cargo receptors. Cleavable vacuolar sorting signals include the NH(2)-terminal propeptide (NTPP) present in sweet potato sporamin (Spo) and the COOH-terminal propeptide (CTPP) present in barley lectin (BL). These two proteins have been found to be transported by different mechanisms to the vacuole. We examined the ability of the vacuolar cargo receptor AtELP to interact with the sorting signals of heterologous and endogenous plant vacuolar proteins in mediating vacuolar transport in Arabidopsis thaliana. AtELP extracted from microsomes was found to interact with the NTPPs of barley aleurain and Spo, but not with the CTPPs of BL or tobacco chitinase, in a pH-dependent and sequence-specific manner. In addition, EM studies revealed the colocalization of AtELP with NTPP-Spo at the Golgi apparatus, but not with BL-CTPP in roots of transgenic Arabidopsis plants. Further, we found that AtELP interacts in a similar manner with the NTPP of the endogenous vacuolar protein AtALEU (Arabidopsis thaliana Aleu), a protein highly homologous to barley aleurain. We hypothesize that AtELP functions as a vacuolar sorting receptor involved in the targeting of NTPP-, but not CTPP-containing proteins in Arabidopsis.
Figures

References
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–441. - PubMed
-
- Bent A.F., Kunkel B.N., Dahlbeck D., Brown K.L., Schmidt R., Giraudat J., Leung J., Staskawicz B.J. RPS2 of Arabidopsis thalianaa leucine-rich repeat class of plant disease resistance genes. Science. 1994;265:1856–1860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

