Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity
- PMID: 10871284
- PMCID: PMC2175140
- DOI: 10.1083/jcb.149.7.1443
Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity
Abstract
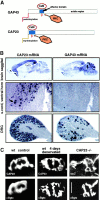
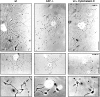
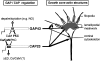
CAP23 is a major cortical cytoskeleton-associated and calmodulin binding protein that is widely and abundantly expressed during development, maintained in selected brain structures in the adult, and reinduced during nerve regeneration. Overexpression of CAP23 in adult neurons of transgenic mice promotes nerve sprouting, but the role of this protein in process outgrowth was not clear. Here, we show that CAP23 is functionally related to GAP43, and plays a critical role to regulate nerve sprouting and the actin cytoskeleton. Knockout mice lacking CAP23 exhibited a pronounced and complex phenotype, including a defect to produce stimulus-induced nerve sprouting at the adult neuromuscular junction. This sprouting deficit was rescued by transgenic overexpression of either CAP23 or GAP43 in adult motoneurons. Knockin mice expressing GAP43 instead of CAP23 were essentially normal, indicating that, although these proteins do not share homologous sequences, GAP43 can functionally substitute for CAP23 in vivo. Cultured sensory neurons lacking CAP23 exhibited striking alterations in neurite outgrowth that were phenocopied by low doses of cytochalasin D. A detailed analysis of such cultures revealed common and unique functions of CAP23 and GAP43 on the actin cytoskeleton and neurite outgrowth. The results provide compelling experimental evidence for the notion that CAP23 and GAP43 are functionally related intrinsic determinants of anatomical plasticity, and suggest that these proteins function by locally promoting subplasmalemmal actin cytoskeleton accumulation.
Figures




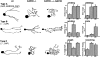
Similar articles
-
GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism.J Cell Biol. 2000 Jun 26;149(7):1455-72. doi: 10.1083/jcb.149.7.1455. J Cell Biol. 2000. PMID: 10871285 Free PMC article.
-
Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction.J Cell Biol. 1997 Feb 10;136(3):679-92. doi: 10.1083/jcb.136.3.679. J Cell Biol. 1997. PMID: 9024697 Free PMC article.
-
Regulation of calcineurin by growth cone calcium waves controls neurite extension.J Neurosci. 2000 Jan 1;20(1):315-25. doi: 10.1523/JNEUROSCI.20-01-00315.2000. J Neurosci. 2000. PMID: 10627609 Free PMC article.
-
New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts.EMBO J. 2001 Aug 15;20(16):4332-6. doi: 10.1093/emboj/20.16.4332. EMBO J. 2001. PMID: 11500359 Free PMC article. Review.
-
Protein kinase C and the regulation of the actin cytoskeleton.Cell Signal. 2006 Mar;18(3):276-84. doi: 10.1016/j.cellsig.2005.07.010. Epub 2005 Aug 16. Cell Signal. 2006. PMID: 16109477 Review.
Cited by
-
Spatially Directed Proteomics of the Human Lens Outer Cortex Reveals an Intermediate Filament Switch Associated With the Remodeling Zone.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4108-14. doi: 10.1167/iovs.16-19791. Invest Ophthalmol Vis Sci. 2016. PMID: 27537260 Free PMC article.
-
GAP-43 and BASP1 in Axon Regeneration: Implications for the Treatment of Neurodegenerative Diseases.Front Cell Dev Biol. 2020 Sep 3;8:567537. doi: 10.3389/fcell.2020.567537. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015061 Free PMC article. Review.
-
Use of Stem Cell Extracellular Vesicles as a "Holistic" Approach to CNS Repair.Front Cell Dev Biol. 2020 Jun 10;8:455. doi: 10.3389/fcell.2020.00455. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32587858 Free PMC article. Review.
-
Prion Protein Deficiency Causes Diverse Proteome Shifts in Cell Models That Escape Detection in Brain Tissue.PLoS One. 2016 Jun 21;11(6):e0156779. doi: 10.1371/journal.pone.0156779. eCollection 2016. PLoS One. 2016. PMID: 27327609 Free PMC article.
-
A high-affinity cocaine binding site associated with the brain acid soluble protein 1.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2200545119. doi: 10.1073/pnas.2200545119. Epub 2022 Apr 11. Proc Natl Acad Sci U S A. 2022. PMID: 35412917 Free PMC article.
References
-
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans. 1995;23:587–591. - PubMed
-
- Aigner, L., S. Arber, J.P. Kapfhammer, T. 0, C. Schneider, F. Botteri, H.-R. Brenner, and P. Caroni. 1995. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 83:269–278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

