Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism
- PMID: 10871641
- PMCID: PMC2229615
- DOI: 10.1085/jgp.116.1.75
Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism
Abstract
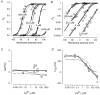
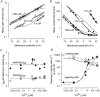
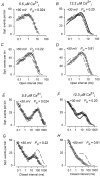
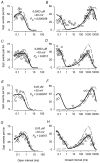
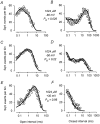
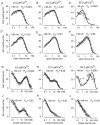
The voltage- and Ca2+-dependent gating mechanism of large-conductance Ca2+-activated K+ (BK) channels from cultured rat skeletal muscle was studied using single-channel analysis. Channel open probability (Po) increased with depolarization, as determined by limiting slope measurements (11 mV per e-fold change in Po; effective gating charge, q(eff), of 2.3 +/- 0.6 e(o)). Estimates of q(eff) were little changed for intracellular Ca2+ (Ca2+(i)) ranging from 0.0003 to 1,024 microM. Increasing Ca2+(i) from 0.03 to 1,024 microM shifted the voltage for half maximal activation (V(1/2)) 175 mV in the hyperpolarizing direction. V(1/2) was independent of Ca2+(i) for Ca2+(i) < or = 0.03 microM, indicating that the channel can be activated in the absence of Ca2+(i). Open and closed dwell-time distributions for data obtained at different Ca2+(i) and voltage, but at the same Po, were different, indicating that the major action of voltage is not through concentrating Ca2+ at the binding sites. The voltage dependence of Po arose from a decrease in the mean closing rate with depolarization (q(eff) = -0.5 e(o)) and an increase in the mean opening rate (q(eff) = 1.8 e(o)), consistent with voltage-dependent steps in both the activation and deactivation pathways. A 50-state two-tiered model with separate voltage- and Ca2+-dependent steps was consistent with the major features of the voltage and Ca2+ dependence of the single-channel kinetics over wide ranges of Ca2+(i) (approximately 0 through 1,024 microM), voltage (+80 to -80 mV), and Po (10(-4) to 0.96). In the model, the voltage dependence of the gating arises mainly from voltage-dependent transitions between closed (C-C) and open (O-O) states, with less voltage dependence for transitions between open and closed states (C-O), and with no voltage dependence for Ca2+-binding and unbinding. The two-tiered model can serve as a working hypothesis for the Ca2+- and voltage-dependent gating of the BK channel.
Figures

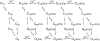




References
-
- Adelman J.P., Sheen E., Kavanaugh M.P., Warren R.A., Wu Y., Lagrutta A., Bond C., North R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Akaike H. A new look at the statistical model identification. IEEE Trans. Auto. Control. 1974;19:716–723.
-
- Almers W. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 1978;82:96–190. - PubMed
-
- Atkinson N.S., Robertson G.A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

