Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts
- PMID: 10877794
- PMCID: PMC92099
- DOI: 10.1128/AEM.66.7.2972-2980.2000
Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts
Abstract
Several in vitro surrogates have been developed as convenient, user-friendly alternatives to mouse infectivity assays for determining the viability of Cryptosporidium parvum oocysts. Such viability assays have been used increasingly to determine oocyst inactivation following treatment with chemical, physical, or environmental stresses. Defining the relationship between in vitro viability assays and oocyst infectivity in susceptible hosts is critical for determining the significance of existing oocyst inactivation data for these in vitro assays and their suitability in future studies. In this study, four viability assays were compared with mouse infectivity assays, using neonatal CD-1 mice. Studies were conducted in the United States and United Kingdom using fresh (<1 month) or environmentally aged (3 months at 4 degrees C) oocysts, which were partially inactivated by ozonation before viability and/or infectivity analyses. High levels of variability were noted within and between the viability and infectivity assays in the U.S. and United Kingdom studies despite rigorous control over oocyst conditions and disinfection experiments. Based on the viability analysis of oocyst subsamples from each ozonation experiment, SYTO-59 assays demonstrated minimal change in oocyst viability, whereas 4',6'-diamidino-2-phenylindole-propidium iodide assays, in vitro excystation, and SYTO-9 assays showed a marginal reduction in oocyst viability. In contrast, the neonatal mouse infectivity assay demonstrated significantly higher levels of oocyst inactivation in the U.S. and United Kingdom experiments. These comparisons illustrate that four in vitro viability assays cannot be used to reliably predict oocyst inactivation following treatment with low levels of ozone. Neonatal mouse infectivity assays should continue to be regarded as a "gold standard" until suitable alternative viability surrogates are identified for disinfection studies.
Figures
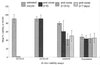

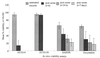
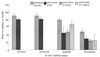

References
-
- Armstrong D C, Casemore D P, Couper A M, Martin A D, Naylor P J. Disinfection of Cryptosporidium parvum with ozone at high concentrations. In: Betts W B, Casemore D P, Fricker C R, Smith H V, Watkins J, editors. Protozoan parasites and water. Cambridge, England: The Royal Society of Chemistry; 1995. pp. 226–229.
-
- Arrowood M J, Donaldson K. Improved purification methods for calf derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol. 1996;43:S89. - PubMed
-
- Belosevic M, Taghi-Kilani R, Guy R A, Neumann N F, Finch G R, Gyürek L L, Liyanage L R J. Vital dye staining of Giardia and Cryptosporidium. Denver, Colo: American Water Works Association; 1997.
-
- Black E K, Finch G R, Taghi-Kilani R, Belosevic M. Comparison of assays for Cryptosporidium parvum oocysts viability after chemical disinfection. FEMS Microbiol Lett. 1996;135:187–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

