Studies on the production of fungal peroxidases in Aspergillus niger
- PMID: 10877800
- PMCID: PMC92105
- DOI: 10.1128/AEM.66.7.3016-3023.2000
Studies on the production of fungal peroxidases in Aspergillus niger
Abstract
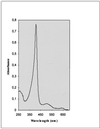
To get insight into the limiting factors existing for the efficient production of fungal peroxidase in filamentous fungi, the expression of the Phanerochaete chrysosporium lignin peroxidase H8 (lipA) and manganese peroxidase (MnP) H4 (mnp1) genes in Aspergillus niger has been studied. For this purpose, a protease-deficient A. niger strain and different expression cassettes have been used. Northern blotting experiments indicated high steady-state mRNA levels for the recombinant genes. Manganese peroxidase was secreted into the culture medium as an active protein. The recombinant protein showed specific activity and a spectrum profile similar to those of the native enzyme, was correctly processed at its N terminus, and had a slightly lower mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Recombinant MnP production could be increased up to 100 mg/liter upon hemoglobin supplementation of the culture medium. Lignin peroxidase was also secreted into the extracellular medium, although the protein was not active, presumably due to incorrect processing of the secreted enzyme. Expression of the lipA and mnp1 genes fused to the A. niger glucoamylase gene did not result in improved production yields.
Figures





References
-
- Aifa M S, Sayadi S, Gargouri A. Heterologous expression of lignin peroxidase of Phanerochaete chrysosporium in Aspergillus niger. Biotechnol Lett. 1999;21:849–853.
-
- Andersen, H. D., E. B. Jensen, and K. G. Welinder. September 1992. A process for producing heme proteins. European Patent Application EP 0 505 311 A2.
-
- Archer D B, Peberdy J F. The molecular biology of secreted enzyme production by fungi. Crit Rev Biotechnol. 1997;17:273–306. - PubMed
-
- Aust S D. Degradation of environmental pollutants by Phanerochaete chrysosporium. Microb Ecol. 1990;20:197–209. - PubMed
-
- Bennett J W, Lasure L L. Growth media. In: Bennett J W, Lasure L L, editors. More gene manipulation in fungi. New York, N.Y: Academic Press, Inc.; 1991. pp. 441–457.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

