The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1
- PMID: 10878097
- PMCID: PMC2269991
- DOI: 10.1111/j.1469-7793.2000.00035.x
The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1
Abstract
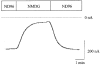
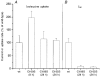
System L is the major Na(+)-independent amino acid transporter of mammalian cells. It is constituted of the type II membrane protein 4F2hc (CD98) which is covalently linked to the polytopic membrane protein LAT1 via a disulfide bridge. The transporter is known to be regulated by the mineral corticoid aldosterone in Xenopus A6 cells. To understand the regulation of the transporter, the 4F2hc/LAT1 heterodimer was functionally expressed in Xenopus laevis oocytes and its transport properties were analysed using flux measurements and the two-electrode voltage-clamp technique. Expression of 4F2hc/LAT1 resulted in a rapid increase in a Na(+)-independent neutral amino acid antiport activity and simultaneously gave rise to a cation conductance. The cation channel was non-rectifying and non-selective, conducting Li(+) > Cs(+) = Na(+) > K(+). After replacement of Na(+) by NMDG, however, the currents were suppressed almost completely. The cation channel was not inhibited by amiloride, Ba2(+), TEA, Hoe293B, flufenamic acid or substrates of the system L amino acid transporter. Significant inhibition, however, was observed in the presence of La3(+), Gd3(+) and quinidine. Channel activity was upregulated by coexpression of 4F2hc/LAT1 with the aldosterone-regulated protein kinase sgk-1. The cation conductance was sensitive to changes in the redox potential, being inhibited following incubation of the oocytes with DTE for 30 min. Mutation of either of the disulfide bridge-constituting cysteines to serine resulted in a loss of ion channel activity whereas amino acid transport was unaffected. It is concluded that the 4F2hc/LAT1 heterodimer regulates a closely associated cation channel or even constitutes a cation channel itself.
Figures
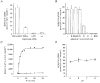
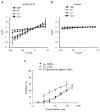

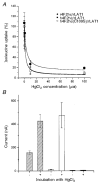
 ), h4F2hc(C109S)/rLAT1 (□) or in uninjected control oocytes (▪). The mean (±
), h4F2hc(C109S)/rLAT1 (□) or in uninjected control oocytes (▪). The mean (±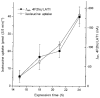
References
-
- Begenisich T. Determining ion channel permeation properties. Methods in Enzymology. 1998;293:383–390. - PubMed
-
- Bielfeld-Ackermann A, Range C, Korbmacher C. Maitotoxin (MTX) activates a nonselective cation channel in Xenopus laevis oocytes. Pflügers Archiv. 1998;436:329–337. - PubMed
-
- Bröer A, Brookes N, Ganapathy V, Dimmer K-S, Wagner CA, Lang F, Bröer S. The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. Journal of Neurochemistry. 1999;73:2184–2194. - PubMed
-
- Bröer S, Bröer A, Hamprecht B. Expression of Na+-independent isoleucine transport activity from rat brain in Xenopus laevis oocytes. Biochimica et Biophysica Acta. 1994;1192:95–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

